Reaction Conditions: Kinetic and Thermodynamic Investigation
VerifiedAdded on 2021/04/17
|6
|1070
|158
Practical Assignment
AI Summary
This assignment investigates the kinetic and thermodynamic reaction conditions in chemical reactions. It explores the influence of temperature on reaction control, differentiating between kinetic and thermodynamic reaction paths based on activation energy and product stability. The experiment involves the competition of furfural and cyclohexanone with semicarbazide to form semicarbazone under varying conditions. The procedure includes dissolving reactants, isolating products through vacuum filtration, and analyzing the effects of temperature and reaction time on product formation and melting points. The results demonstrate that increased temperature impacts the reaction and product mass. The assignment concludes that the competition of reactants can result in kinetic products, with temperature increases influencing the reaction and leading to increased mass of products with increased melting points. Errors are acknowledged and minimized through equipment calibration.

TITLE: INVESTIGATING KINETIC AND THERMODYNAMIC REACTION CONDITIONS
FOR REACTIONS
Objectives
Investigating kinetic and thermodynamic control in chemical reactions
Investigating the effect of temperature on control of chemical reactions
Introduction and background
Chemical reactants can follow different to produce the products. The paths followed are usually
influenced by different conditions. The major difference in the type of reaction path followed is
the amount of reactivation energy required to achieve the end results (Marini, 2007). Two major
reaction paths include the kinetic and thermodynamic reaction paths. When results are formed
quickly, it is assumed that less activation energy was used to complete the reaction. The reaction
is therefore considered to have been controlled through the kinetic path (Brown et al., 2015).
Therefore, the kinetic path is the path which requires less activation energy to complete the
reaction. On the other hand, under the thermodynamic path, the reactants are assumed to be at
equilibrium and the results to be more stable products which always dominate. Under the two
reaction paths, the product stability is different. The thermodynamic products are more stable
than the kinetic products. Graphically, it is always seen that the thermodynamic products are
lower than the kinetic products (Nishinaga et al., 2012). This is because the thermodynamic
products are usually at lower energy than the kinetic products. In addition, temperatures are able
to favor the thermodynamic reaction path.
In addition, these concepts are also applied when the reactant compete to form two related
products from a single reactant. This is where the reactants are forcing to follow either the
FOR REACTIONS
Objectives
Investigating kinetic and thermodynamic control in chemical reactions
Investigating the effect of temperature on control of chemical reactions
Introduction and background
Chemical reactants can follow different to produce the products. The paths followed are usually
influenced by different conditions. The major difference in the type of reaction path followed is
the amount of reactivation energy required to achieve the end results (Marini, 2007). Two major
reaction paths include the kinetic and thermodynamic reaction paths. When results are formed
quickly, it is assumed that less activation energy was used to complete the reaction. The reaction
is therefore considered to have been controlled through the kinetic path (Brown et al., 2015).
Therefore, the kinetic path is the path which requires less activation energy to complete the
reaction. On the other hand, under the thermodynamic path, the reactants are assumed to be at
equilibrium and the results to be more stable products which always dominate. Under the two
reaction paths, the product stability is different. The thermodynamic products are more stable
than the kinetic products. Graphically, it is always seen that the thermodynamic products are
lower than the kinetic products (Nishinaga et al., 2012). This is because the thermodynamic
products are usually at lower energy than the kinetic products. In addition, temperatures are able
to favor the thermodynamic reaction path.
In addition, these concepts are also applied when the reactant compete to form two related
products from a single reactant. This is where the reactants are forcing to follow either the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

kinetic path or the thermodynamic path to form the products (Chen, 2014). As in the following
reaction, the reactant will be competing to form the products through the two paths. The use of
aldehyde or ketone together with semicarbazide to form semicarbazone is one of such reaction as
illustrated below;
RCOR’ + H2NNHCONH2 RR’C=NNHCONH2 + H2O
Semicarbazide semicarbazone
This experiment will allow furfural and cyclohexanone to compete for semicarbazide under
different conditions in order to form different semicarbazone at different rates and with different
stabilities.
Experiment procedure
A (i)
1. Dissolve 1.7g 15.2 mmol semicarbazide HCL in a solution of 3g sodium bicarbonate in
35 ml of water.
2. When the effervescence seases, add 1.5ml 15.3mmol cyclohexanone.
3. Shake the mixture for 10 min. isolate the product through vacuum filtration.
4. Wash the products with ice water and allow them to dry.
5. Label and record the weight and melting point of the products
A (ii)
Repeating the above process with 1.3 ml 15.7 mmol distilled furfural. Set the reaction time to be
30 min on a steam bath. Label the results and record the weight and melting point.
reaction, the reactant will be competing to form the products through the two paths. The use of
aldehyde or ketone together with semicarbazide to form semicarbazone is one of such reaction as
illustrated below;
RCOR’ + H2NNHCONH2 RR’C=NNHCONH2 + H2O
Semicarbazide semicarbazone
This experiment will allow furfural and cyclohexanone to compete for semicarbazide under
different conditions in order to form different semicarbazone at different rates and with different
stabilities.
Experiment procedure
A (i)
1. Dissolve 1.7g 15.2 mmol semicarbazide HCL in a solution of 3g sodium bicarbonate in
35 ml of water.
2. When the effervescence seases, add 1.5ml 15.3mmol cyclohexanone.
3. Shake the mixture for 10 min. isolate the product through vacuum filtration.
4. Wash the products with ice water and allow them to dry.
5. Label and record the weight and melting point of the products
A (ii)
Repeating the above process with 1.3 ml 15.7 mmol distilled furfural. Set the reaction time to be
30 min on a steam bath. Label the results and record the weight and melting point.

B (i) competitive reactions
As per procedure in A (i) above, semicarbazonewas prepared which is a mixture of 0.8 ml
furfural, 1ml cyclohexanone and 1g semicarbazide HCL dissolved in 2.0g sodium bicarbonate in
25ml water. Shake the mixture until precipitation forms. The product were isolated through
vacuum filtration, washed with ice water and allowed to dry. Later they were labeled and their
weight and MP recorded.
B(ii)
The process in B(i) was repeated and the heating the reaction mixture on steam bath was carried
for 1.5 Hrs. the products were labeled and their weight and MP recorded.
C(i) Interconversion attempts
1. A mixture of 0.5g cyclohexanone semicarbazone and 0.4ml furfural in 10ml of water was
boiled for 30 minute and allowed to cool.
2. The precipitation of semicarbazone was collected through vacuum filtration.
3. The products were washed with little ice water and allowed to dry.
4. Later they were labeled and their weight and MP recorded.
C(ii)
The procedure in C(ii) above was repeated but the reactant used were 0.5g furfural
semicarbazone and 0.4ml cyclohexanone. Later the products were labeled and their weight and
MP recorded.
692182
As per procedure in A (i) above, semicarbazonewas prepared which is a mixture of 0.8 ml
furfural, 1ml cyclohexanone and 1g semicarbazide HCL dissolved in 2.0g sodium bicarbonate in
25ml water. Shake the mixture until precipitation forms. The product were isolated through
vacuum filtration, washed with ice water and allowed to dry. Later they were labeled and their
weight and MP recorded.
B(ii)
The process in B(i) was repeated and the heating the reaction mixture on steam bath was carried
for 1.5 Hrs. the products were labeled and their weight and MP recorded.
C(i) Interconversion attempts
1. A mixture of 0.5g cyclohexanone semicarbazone and 0.4ml furfural in 10ml of water was
boiled for 30 minute and allowed to cool.
2. The precipitation of semicarbazone was collected through vacuum filtration.
3. The products were washed with little ice water and allowed to dry.
4. Later they were labeled and their weight and MP recorded.
C(ii)
The procedure in C(ii) above was repeated but the reactant used were 0.5g furfural
semicarbazone and 0.4ml cyclohexanone. Later the products were labeled and their weight and
MP recorded.
692182
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
(e) Results and Discussion
Results
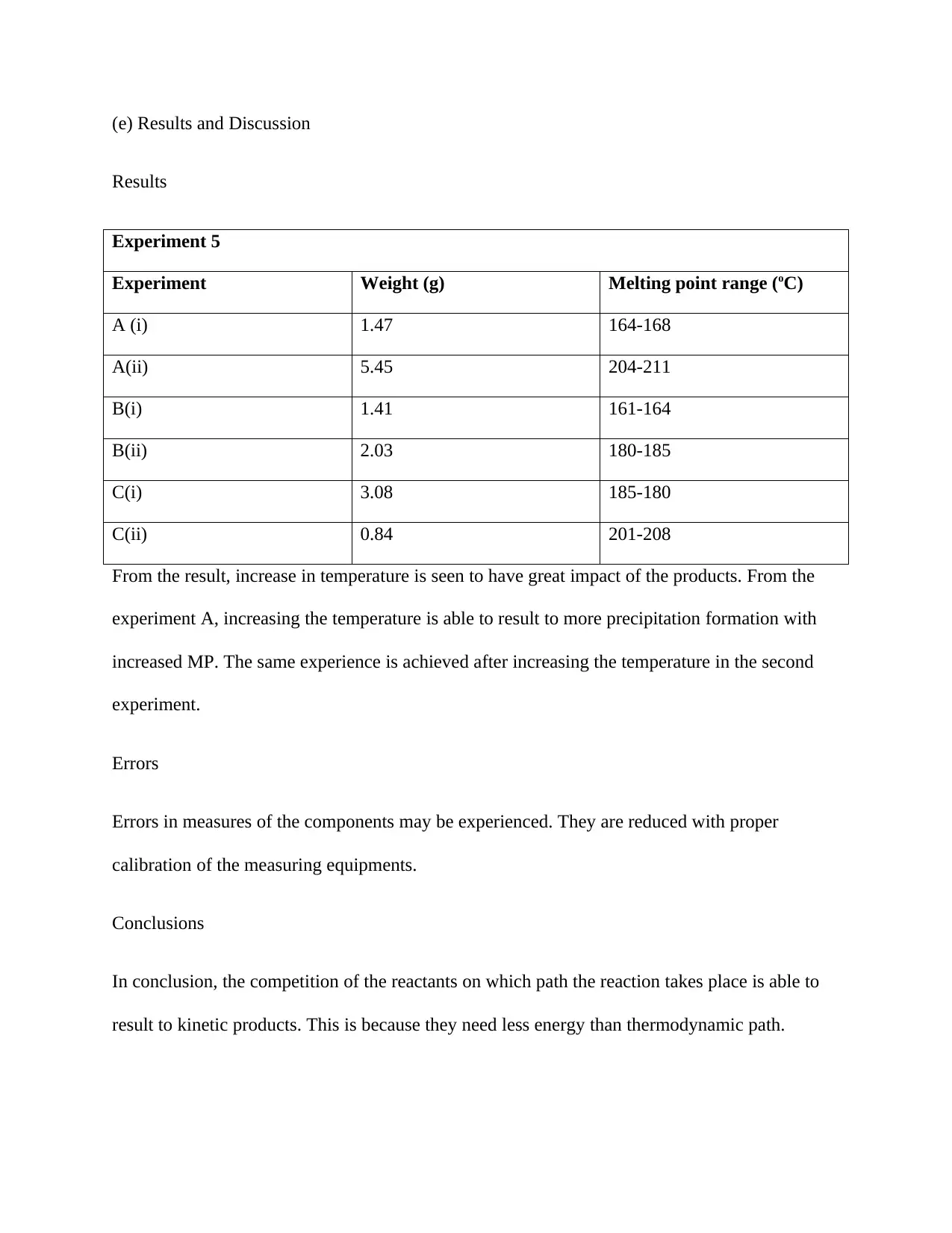
Experiment 5
Experiment Weight (g) Melting point range (oC)
A (i) 1.47 164-168
A(ii) 5.45 204-211
B(i) 1.41 161-164
B(ii) 2.03 180-185
C(i) 3.08 185-180
C(ii) 0.84 201-208
From the result, increase in temperature is seen to have great impact of the products. From the
experiment A, increasing the temperature is able to result to more precipitation formation with
increased MP. The same experience is achieved after increasing the temperature in the second
experiment.
Errors
Errors in measures of the components may be experienced. They are reduced with proper
calibration of the measuring equipments.
Conclusions
In conclusion, the competition of the reactants on which path the reaction takes place is able to
result to kinetic products. This is because they need less energy than thermodynamic path.
Results
Experiment 5
Experiment Weight (g) Melting point range (oC)
A (i) 1.47 164-168
A(ii) 5.45 204-211
B(i) 1.41 161-164
B(ii) 2.03 180-185
C(i) 3.08 185-180
C(ii) 0.84 201-208
From the result, increase in temperature is seen to have great impact of the products. From the
experiment A, increasing the temperature is able to result to more precipitation formation with
increased MP. The same experience is achieved after increasing the temperature in the second
experiment.
Errors
Errors in measures of the components may be experienced. They are reduced with proper
calibration of the measuring equipments.
Conclusions
In conclusion, the competition of the reactants on which path the reaction takes place is able to
result to kinetic products. This is because they need less energy than thermodynamic path.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Temperature increase is able to influence the reaction and leads to increased mass of products
with increased MP.
Under kinetic reaction, P1 product will dominate while P2 will dominate under thermodynamic
reaction. In energy diagram 2, P1 will dominate under kinetic and thermodynamic reactions.
with increased MP.
Under kinetic reaction, P1 product will dominate while P2 will dominate under thermodynamic
reaction. In energy diagram 2, P1 will dominate under kinetic and thermodynamic reactions.

References
Brown, T. L., LeMay, H. E., Bursten, B. E., Murphy, C. J., Woodward, P., & Stoltzfus, M. W.
(2015). Chemistry: The central science. Boston: Pearson.
Chen, Y. (2014). Kinetic competition growth mechanism and phase manipulation of silicide
nanowires in solid state reaction. Los Angeles: University of California, Los Angeles.
Marini, L. (2007). Geological sequestration of carbon dioxide: Thermodynamics, kinetics, and
reaction path modeling. Amsterdam: Elsevier.
Nishinaga, T., Nishioka, K., Harada, J., Sasaki, A., & Takei, H. (2012). Advances in the
Understanding of Crystal Growth Mechanisms. Burlington: Elsevier Science.
Brown, T. L., LeMay, H. E., Bursten, B. E., Murphy, C. J., Woodward, P., & Stoltzfus, M. W.
(2015). Chemistry: The central science. Boston: Pearson.
Chen, Y. (2014). Kinetic competition growth mechanism and phase manipulation of silicide
nanowires in solid state reaction. Los Angeles: University of California, Los Angeles.
Marini, L. (2007). Geological sequestration of carbon dioxide: Thermodynamics, kinetics, and
reaction path modeling. Amsterdam: Elsevier.
Nishinaga, T., Nishioka, K., Harada, J., Sasaki, A., & Takei, H. (2012). Advances in the
Understanding of Crystal Growth Mechanisms. Burlington: Elsevier Science.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



