Kinetics and Chemical Equilibrium Lab: Course Code Experiment
VerifiedAdded on 2023/04/21
|8
|972
|186
Practical Assignment
AI Summary
This practical assignment presents a laboratory experiment focused on kinetics and chemical equilibrium. The experiment investigates the reaction rate of brass with boiling acid and base, noting the differences in reaction rates and mass loss. Safety precautions for handling acids are emphasized, including proper dilution techniques and the use of safety glasses. Additionally, the experiment explores the reaction between hydrochloric acid and sodium thiosulfate, observing the formation of a white precipitate and measuring the time taken for printed words to disappear as an indicator of reaction rate. The report includes data collection, graphical analysis, and interpretation of results, highlighting the effect of concentration on reaction rate. The document concludes with relevant references.

Running Head: KINETICS AND CHEMICAL EQUILIBRIUM 1
Kinetics and Chemical Equilibrium
Student Name
Date
Institution Affiliate
Kinetics and Chemical Equilibrium
Student Name
Date
Institution Affiliate
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
KINETICS AND CHEMICAL EQUILIBRIUM
1. A Laboratory experiment was conducted to illustrate the reaction rate of brass with boiling
acid and boiling base. Brass is an alloy of copper and zinc. The experimental set up was
done and the results were recorded within 12 hours. Graphs for mass of brass against
time for boiling acid and boiling base were drawn and after data was collected. Brass
reacts with both boiling acid and boiling base as shown in the graph. The only difference
is the rate of reaction. Two identical pieces of brass were placed in two different boiling
liquids for 12 hours. Using the provided information:
a. One similarity in the change in mass of the brass in both liquids is that Brass reacts with
both acid and base but the reaction rate with acid is more vigorous as compared to base.
b. Two ways in which the loss of mass shown in graph A is different from that shown in
graph B
i. In graph A, there is uniform mass loss meaning brass is reacting with the boiling acid
with the 12 hours while according to graph B, the mass loss stopped after some time
indicating no further reaction is taking place.
ii. Secondly, there is significant mass loss in graph A while the mass loss in graph B is
insignificant meaning reaction of brass with boiling base is a slow reaction or equilibrium
has been attained.
c. Three different safety precautions that are needed in doing this investigation
i. Acid solutions should be handled carefully as acids may be volatile. Safety glasses must
be worn.
KINETICS AND CHEMICAL EQUILIBRIUM
1. A Laboratory experiment was conducted to illustrate the reaction rate of brass with boiling
acid and boiling base. Brass is an alloy of copper and zinc. The experimental set up was
done and the results were recorded within 12 hours. Graphs for mass of brass against
time for boiling acid and boiling base were drawn and after data was collected. Brass
reacts with both boiling acid and boiling base as shown in the graph. The only difference
is the rate of reaction. Two identical pieces of brass were placed in two different boiling
liquids for 12 hours. Using the provided information:
a. One similarity in the change in mass of the brass in both liquids is that Brass reacts with
both acid and base but the reaction rate with acid is more vigorous as compared to base.
b. Two ways in which the loss of mass shown in graph A is different from that shown in
graph B
i. In graph A, there is uniform mass loss meaning brass is reacting with the boiling acid
with the 12 hours while according to graph B, the mass loss stopped after some time
indicating no further reaction is taking place.
ii. Secondly, there is significant mass loss in graph A while the mass loss in graph B is
insignificant meaning reaction of brass with boiling base is a slow reaction or equilibrium
has been attained.
c. Three different safety precautions that are needed in doing this investigation
i. Acid solutions should be handled carefully as acids may be volatile. Safety glasses must
be worn.

3
KINETICS AND CHEMICAL EQUILIBRIUM
ii. While diluting the acid, it’s the acid that needs to be poured into the water but not the
water being poured into the acid.
iii. Within the 12 hours, the temperatures of the two boiling liquids should be kept the same
for best results to be obtained.
2. Hydrochloric acid (HCL) reacts with Sodium Thiosulfate solution (NA2S2O3(aq)) to form a
white precipitate. The main objective of the experiment is to investigate the effect of
concentration on the rate of reaction between Hydrochloric acid and Sodium Thiosulfate
solution. Hydrochloric acid reacts with Sodium Thiosulfate to produce sulphur, sulphur
dioxide and water according to the below equation.
Na2S2O3(aq) + 2HCL S(s) +2NACL(aq) +SO2(g) +H2O(l)
The Sulphur dioxide being a highly soluble gas dissolves totally in the aqueous solution. Sulphur
since its insoluble remains in the mixture as a pale yellow or white precipitate and gives a milky
appearance which makes the solution milky and thus making the printed words on the paper to
disappear. Before the reaction starts, the printed words on the piece of paper are clearly visible
when viewed on top of the conical flask. However, as the reaction proceeds, sulphur precipitates
make the solution opaque and eventually the printed words disappear completely.
The time for the reaction varies with amount of reactants used. The time for the printed sheet to
completely disappear indicate how fast the reaction has taken place. Five tests were carried out
using different volumes of sodium thiosulfate, distilled water and hydrochloric acid as follows.
KINETICS AND CHEMICAL EQUILIBRIUM
ii. While diluting the acid, it’s the acid that needs to be poured into the water but not the
water being poured into the acid.
iii. Within the 12 hours, the temperatures of the two boiling liquids should be kept the same
for best results to be obtained.
2. Hydrochloric acid (HCL) reacts with Sodium Thiosulfate solution (NA2S2O3(aq)) to form a
white precipitate. The main objective of the experiment is to investigate the effect of
concentration on the rate of reaction between Hydrochloric acid and Sodium Thiosulfate
solution. Hydrochloric acid reacts with Sodium Thiosulfate to produce sulphur, sulphur
dioxide and water according to the below equation.
Na2S2O3(aq) + 2HCL S(s) +2NACL(aq) +SO2(g) +H2O(l)
The Sulphur dioxide being a highly soluble gas dissolves totally in the aqueous solution. Sulphur
since its insoluble remains in the mixture as a pale yellow or white precipitate and gives a milky
appearance which makes the solution milky and thus making the printed words on the paper to
disappear. Before the reaction starts, the printed words on the piece of paper are clearly visible
when viewed on top of the conical flask. However, as the reaction proceeds, sulphur precipitates
make the solution opaque and eventually the printed words disappear completely.
The time for the reaction varies with amount of reactants used. The time for the printed sheet to
completely disappear indicate how fast the reaction has taken place. Five tests were carried out
using different volumes of sodium thiosulfate, distilled water and hydrochloric acid as follows.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
KINETICS AND CHEMICAL EQUILIBRIUM
Test 1 was conducted using 60 cm3 of aqueous sodium thiosulfate, 10 cm3 of hydrochloric acid
and the time taken for the printed words to disappear was recorded.
Test 2 was conducted using 50 cm3 of aqueous sodium thiosulfate, followed by 10 cm3 of
distilled water. 10 cm3 of hydrochloric acid was then added to the solution and time taken
recorded.
Test 3 was conducted using 45 cm3 of Na2S2O3(aq), 25 cm3 of distilled water then 10 cm3 of HCl
was added. The time was recorded.
Test 4 was done using 35 cm3 of Na2S2O3(aq) and 15 cm3 of distilled water followed by 10 cm3 of
hydrochloric acid.
Test 5 was conducted using 10 cm3 of aqueous sodium thiosulfate and 20 cm3 of distilled water.
Time taken for each test was recorded as shown below
KINETICS AND CHEMICAL EQUILIBRIUM
Test 1 was conducted using 60 cm3 of aqueous sodium thiosulfate, 10 cm3 of hydrochloric acid
and the time taken for the printed words to disappear was recorded.
Test 2 was conducted using 50 cm3 of aqueous sodium thiosulfate, followed by 10 cm3 of
distilled water. 10 cm3 of hydrochloric acid was then added to the solution and time taken
recorded.
Test 3 was conducted using 45 cm3 of Na2S2O3(aq), 25 cm3 of distilled water then 10 cm3 of HCl
was added. The time was recorded.
Test 4 was done using 35 cm3 of Na2S2O3(aq) and 15 cm3 of distilled water followed by 10 cm3 of
hydrochloric acid.
Test 5 was conducted using 10 cm3 of aqueous sodium thiosulfate and 20 cm3 of distilled water.
Time taken for each test was recorded as shown below
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
5
KINETICS AND CHEMICAL EQUILIBRIUM
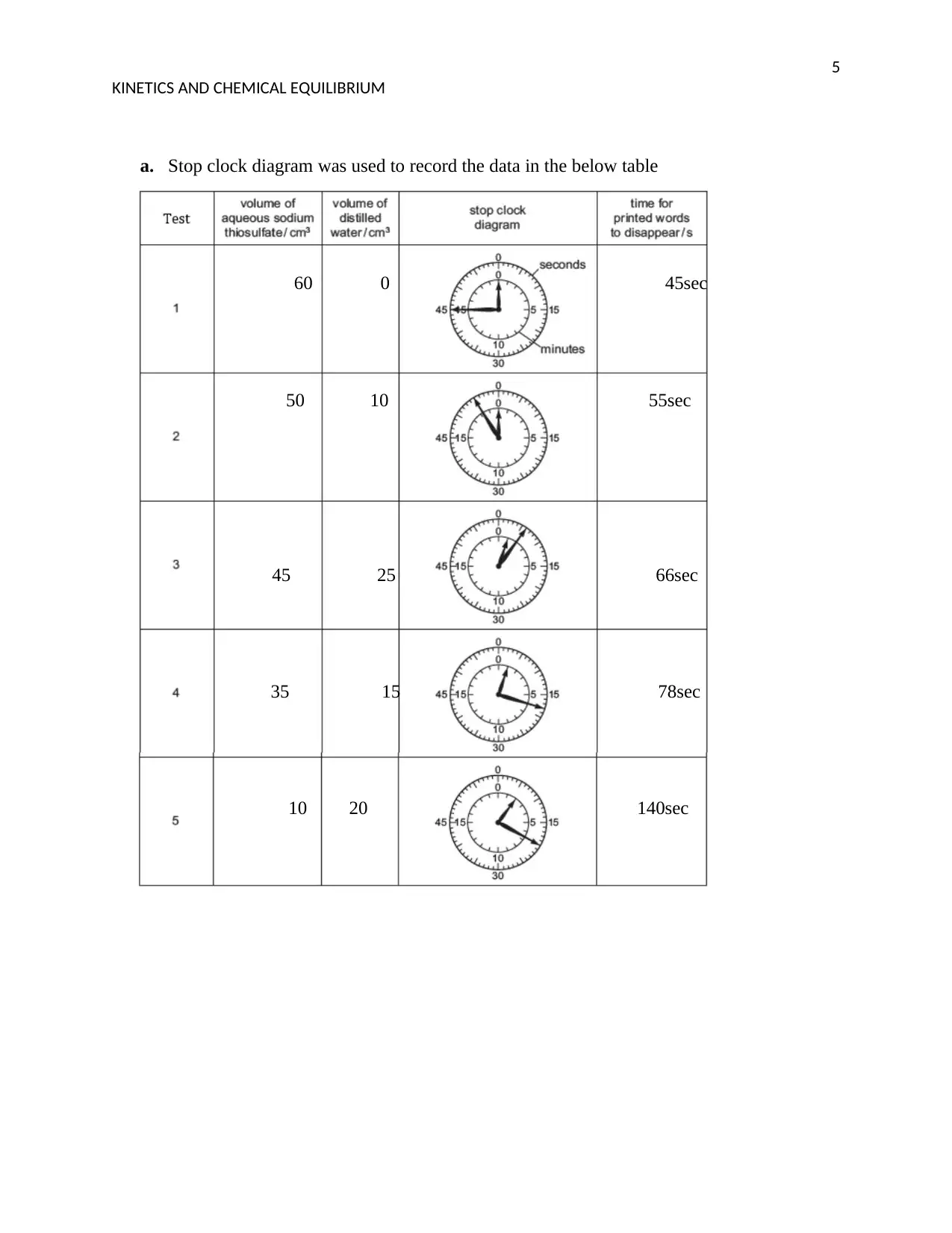
a. Stop clock diagram was used to record the data in the below table
60 0 45sec
50 10 55sec
45 25 66sec
35 15 78sec
10 20 140sec
KINETICS AND CHEMICAL EQUILIBRIUM
a. Stop clock diagram was used to record the data in the below table
60 0 45sec
50 10 55sec
45 25 66sec
35 15 78sec
10 20 140sec
6
KINETICS AND CHEMICAL EQUILIBRIUM
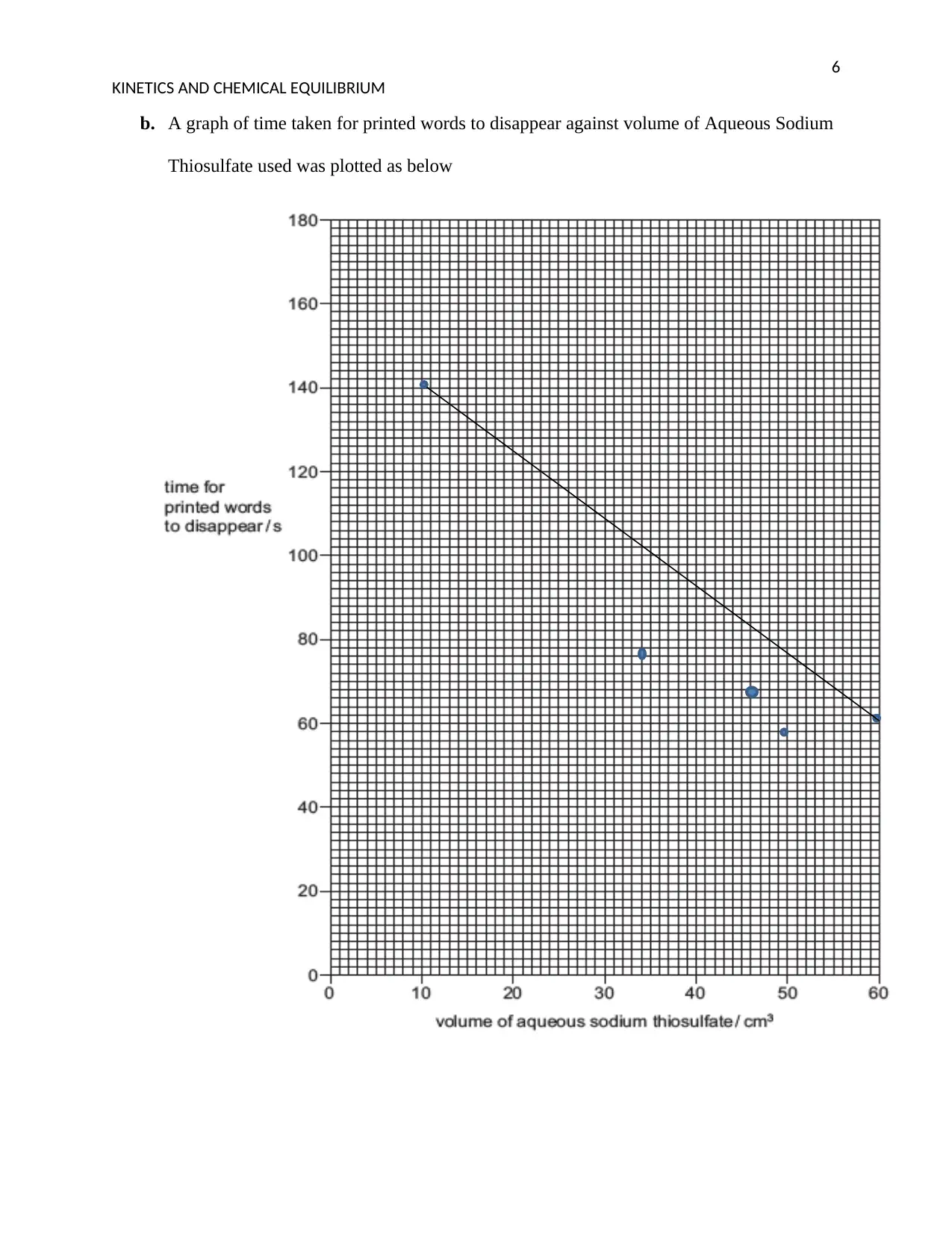
b. A graph of time taken for printed words to disappear against volume of Aqueous Sodium
Thiosulfate used was plotted as below
KINETICS AND CHEMICAL EQUILIBRIUM
b. A graph of time taken for printed words to disappear against volume of Aqueous Sodium
Thiosulfate used was plotted as below
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
KINETICS AND CHEMICAL EQUILIBRIUM
c. The time that will be taken for the printed words to disappear when 15 cm3 of sodium
thiosulfate and 15 cm3 of distilled water are used will be longer. This is so because the
concentration of the sodium will be less due to equal amount of water being used.
d. The rate of reaction was greatest in test 1. This is because of the concentration of the
sodium thiosulfate or the volume of the sodium thiosulfate used.
KINETICS AND CHEMICAL EQUILIBRIUM
c. The time that will be taken for the printed words to disappear when 15 cm3 of sodium
thiosulfate and 15 cm3 of distilled water are used will be longer. This is so because the
concentration of the sodium will be less due to equal amount of water being used.
d. The rate of reaction was greatest in test 1. This is because of the concentration of the
sodium thiosulfate or the volume of the sodium thiosulfate used.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
KINETICS AND CHEMICAL EQUILIBRIUM
1. Polunin, A. V., et al. "Electrochemical studies of the kinetics and mechanism of
brass dezincification." Electrochimica Acta27.4 (1982): 467-475.
2. Espenson, J.H., 1995. Chemical kinetics and reaction mechanisms (Vol. 102).
New York:
KINETICS AND CHEMICAL EQUILIBRIUM
1. Polunin, A. V., et al. "Electrochemical studies of the kinetics and mechanism of
brass dezincification." Electrochimica Acta27.4 (1982): 467-475.
2. Espenson, J.H., 1995. Chemical kinetics and reaction mechanisms (Vol. 102).
New York:
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


