Environmental Impact: Lead Acid Battery versus Hydrogen Fuel Cell
VerifiedAdded on 2023/06/08
|4
|1149
|151
Report
AI Summary
This report provides a comparative analysis of lead-acid batteries and hydrogen fuel cells, focusing on their advantages, disadvantages, and environmental impact. It discusses the reliability, efficiency, and environmental friendliness of hydrogen fuel cells, highlighting their use of hydrogen to produce electricity and water. The report also addresses the environmental concerns associated with lead-acid batteries, particularly the dangers of lead poisoning and the challenges of proper disposal. Worker safety issues related to both technologies are examined, including the risks of hydrogen leakage and lead exposure. The report further compares the power output and disposal methods of both power sources, emphasizing the recyclability of hydrogen fuel cell byproducts and the toxicity of lead-acid battery waste. Ultimately, the report aims to provide a comprehensive overview of the two technologies to inform decision-making regarding their use and management.

Running head: EXTENDED RESPONSE TASK 1
Extended Response Task on Lead Acid Battery versus Hydrogen Fuel Cell
Firstname Lastname
Name of Institution
Extended Response Task on Lead Acid Battery versus Hydrogen Fuel Cell
Firstname Lastname
Name of Institution
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
EXTENDED RESPONSE TASK
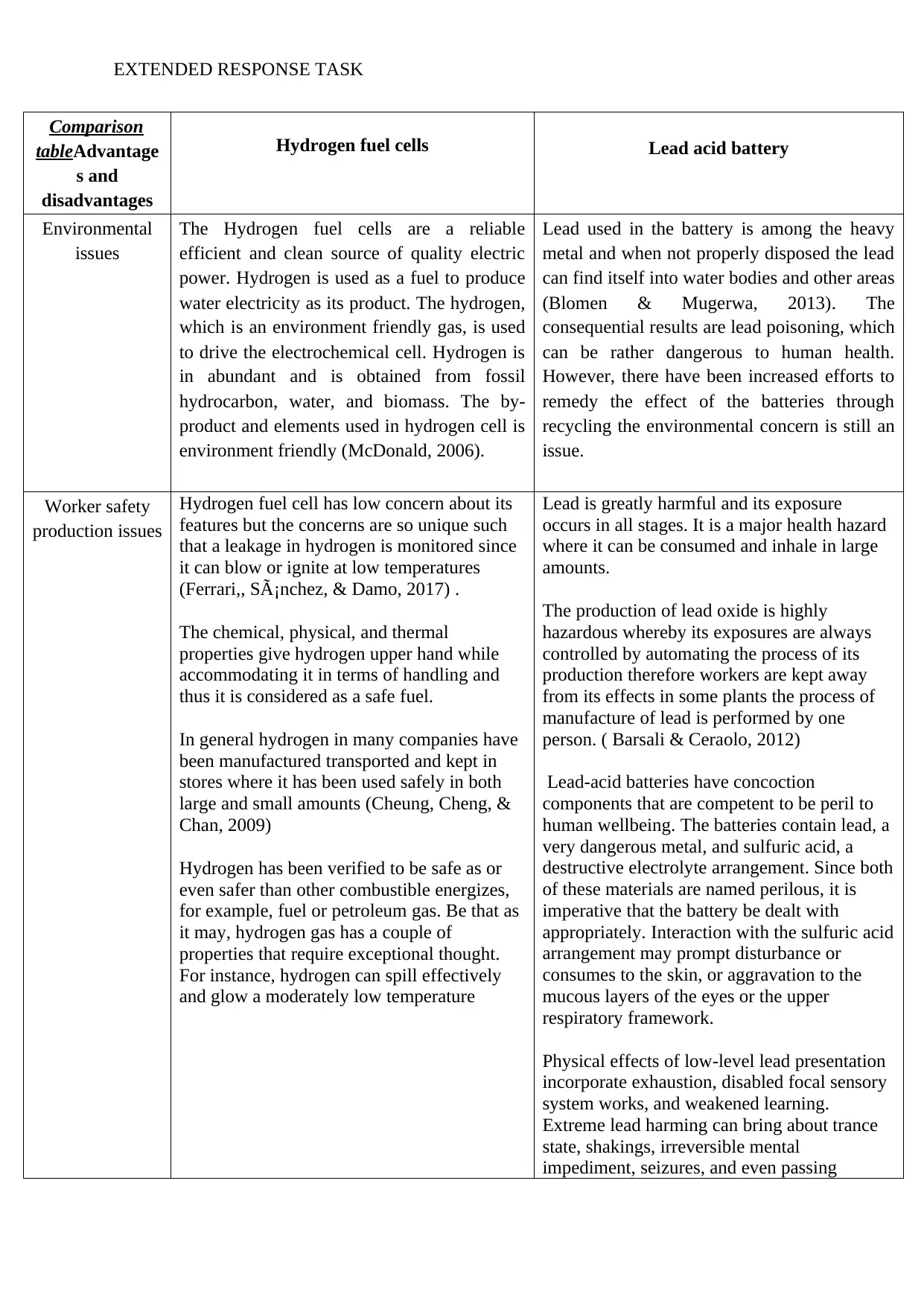
Comparison
tableAdvantage
s and
disadvantages
Hydrogen fuel cells Lead acid battery
Environmental
issues
The Hydrogen fuel cells are a reliable
efficient and clean source of quality electric
power. Hydrogen is used as a fuel to produce
water electricity as its product. The hydrogen,
which is an environment friendly gas, is used
to drive the electrochemical cell. Hydrogen is
in abundant and is obtained from fossil
hydrocarbon, water, and biomass. The by-
product and elements used in hydrogen cell is
environment friendly (McDonald, 2006).
Lead used in the battery is among the heavy
metal and when not properly disposed the lead
can find itself into water bodies and other areas
(Blomen & Mugerwa, 2013). The
consequential results are lead poisoning, which
can be rather dangerous to human health.
However, there have been increased efforts to
remedy the effect of the batteries through
recycling the environmental concern is still an
issue.
Worker safety
production issues
Hydrogen fuel cell has low concern about its
features but the concerns are so unique such
that a leakage in hydrogen is monitored since
it can blow or ignite at low temperatures
(Ferrari,, Sánchez, & Damo, 2017) .
The chemical, physical, and thermal
properties give hydrogen upper hand while
accommodating it in terms of handling and
thus it is considered as a safe fuel.
In general hydrogen in many companies have
been manufactured transported and kept in
stores where it has been used safely in both
large and small amounts (Cheung, Cheng, &
Chan, 2009)
Hydrogen has been verified to be safe as or
even safer than other combustible energizes,
for example, fuel or petroleum gas. Be that as
it may, hydrogen gas has a couple of
properties that require exceptional thought.
For instance, hydrogen can spill effectively
and glow a moderately low temperature
Lead is greatly harmful and its exposure
occurs in all stages. It is a major health hazard
where it can be consumed and inhale in large
amounts.
The production of lead oxide is highly
hazardous whereby its exposures are always
controlled by automating the process of its
production therefore workers are kept away
from its effects in some plants the process of
manufacture of lead is performed by one
person. ( Barsali & Ceraolo, 2012)
Lead-acid batteries have concoction
components that are competent to be peril to
human wellbeing. The batteries contain lead, a
very dangerous metal, and sulfuric acid, a
destructive electrolyte arrangement. Since both
of these materials are named perilous, it is
imperative that the battery be dealt with
appropriately. Interaction with the sulfuric acid
arrangement may prompt disturbance or
consumes to the skin, or aggravation to the
mucous layers of the eyes or the upper
respiratory framework.
Physical effects of low-level lead presentation
incorporate exhaustion, disabled focal sensory
system works, and weakened learning.
Extreme lead harming can bring about trance
state, shakings, irreversible mental
impediment, seizures, and even passing
Comparison
tableAdvantage
s and
disadvantages
Hydrogen fuel cells Lead acid battery
Environmental
issues
The Hydrogen fuel cells are a reliable
efficient and clean source of quality electric
power. Hydrogen is used as a fuel to produce
water electricity as its product. The hydrogen,
which is an environment friendly gas, is used
to drive the electrochemical cell. Hydrogen is
in abundant and is obtained from fossil
hydrocarbon, water, and biomass. The by-
product and elements used in hydrogen cell is
environment friendly (McDonald, 2006).
Lead used in the battery is among the heavy
metal and when not properly disposed the lead
can find itself into water bodies and other areas
(Blomen & Mugerwa, 2013). The
consequential results are lead poisoning, which
can be rather dangerous to human health.
However, there have been increased efforts to
remedy the effect of the batteries through
recycling the environmental concern is still an
issue.
Worker safety
production issues
Hydrogen fuel cell has low concern about its
features but the concerns are so unique such
that a leakage in hydrogen is monitored since
it can blow or ignite at low temperatures
(Ferrari,, Sánchez, & Damo, 2017) .
The chemical, physical, and thermal
properties give hydrogen upper hand while
accommodating it in terms of handling and
thus it is considered as a safe fuel.
In general hydrogen in many companies have
been manufactured transported and kept in
stores where it has been used safely in both
large and small amounts (Cheung, Cheng, &
Chan, 2009)
Hydrogen has been verified to be safe as or
even safer than other combustible energizes,
for example, fuel or petroleum gas. Be that as
it may, hydrogen gas has a couple of
properties that require exceptional thought.
For instance, hydrogen can spill effectively
and glow a moderately low temperature
Lead is greatly harmful and its exposure
occurs in all stages. It is a major health hazard
where it can be consumed and inhale in large
amounts.
The production of lead oxide is highly
hazardous whereby its exposures are always
controlled by automating the process of its
production therefore workers are kept away
from its effects in some plants the process of
manufacture of lead is performed by one
person. ( Barsali & Ceraolo, 2012)
Lead-acid batteries have concoction
components that are competent to be peril to
human wellbeing. The batteries contain lead, a
very dangerous metal, and sulfuric acid, a
destructive electrolyte arrangement. Since both
of these materials are named perilous, it is
imperative that the battery be dealt with
appropriately. Interaction with the sulfuric acid
arrangement may prompt disturbance or
consumes to the skin, or aggravation to the
mucous layers of the eyes or the upper
respiratory framework.
Physical effects of low-level lead presentation
incorporate exhaustion, disabled focal sensory
system works, and weakened learning.
Extreme lead harming can bring about trance
state, shakings, irreversible mental
impediment, seizures, and even passing
EXTENDED RESPONSE TASK
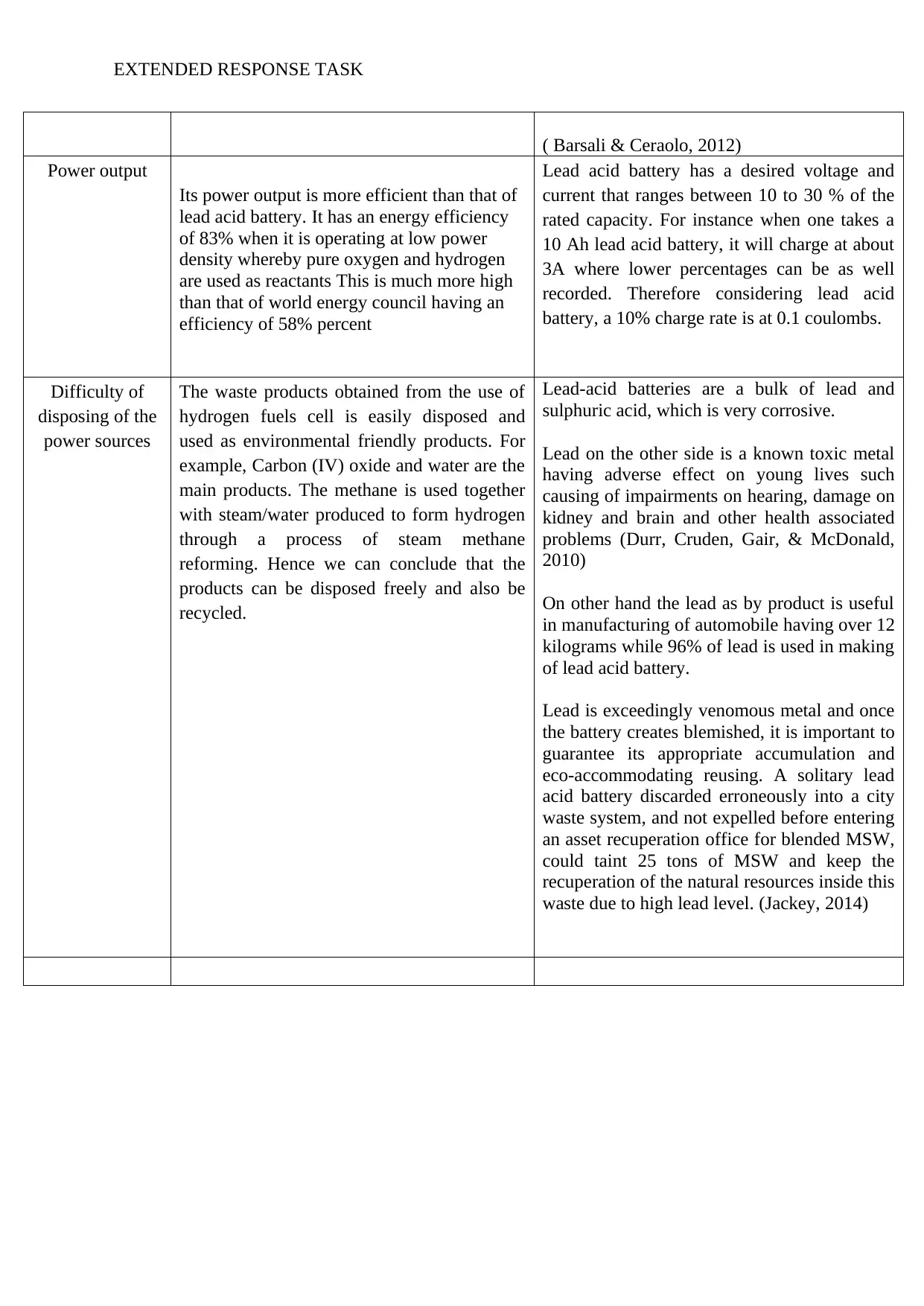
( Barsali & Ceraolo, 2012)
Power output
Its power output is more efficient than that of
lead acid battery. It has an energy efficiency
of 83% when it is operating at low power
density whereby pure oxygen and hydrogen
are used as reactants This is much more high
than that of world energy council having an
efficiency of 58% percent
Lead acid battery has a desired voltage and
current that ranges between 10 to 30 % of the
rated capacity. For instance when one takes a
10 Ah lead acid battery, it will charge at about
3A where lower percentages can be as well
recorded. Therefore considering lead acid
battery, a 10% charge rate is at 0.1 coulombs.
Difficulty of
disposing of the
power sources
The waste products obtained from the use of
hydrogen fuels cell is easily disposed and
used as environmental friendly products. For
example, Carbon (IV) oxide and water are the
main products. The methane is used together
with steam/water produced to form hydrogen
through a process of steam methane
reforming. Hence we can conclude that the
products can be disposed freely and also be
recycled.
Lead-acid batteries are a bulk of lead and
sulphuric acid, which is very corrosive.
Lead on the other side is a known toxic metal
having adverse effect on young lives such
causing of impairments on hearing, damage on
kidney and brain and other health associated
problems (Durr, Cruden, Gair, & McDonald,
2010)
On other hand the lead as by product is useful
in manufacturing of automobile having over 12
kilograms while 96% of lead is used in making
of lead acid battery.
Lead is exceedingly venomous metal and once
the battery creates blemished, it is important to
guarantee its appropriate accumulation and
eco-accommodating reusing. A solitary lead
acid battery discarded erroneously into a city
waste system, and not expelled before entering
an asset recuperation office for blended MSW,
could taint 25 tons of MSW and keep the
recuperation of the natural resources inside this
waste due to high lead level. (Jackey, 2014)
( Barsali & Ceraolo, 2012)
Power output
Its power output is more efficient than that of
lead acid battery. It has an energy efficiency
of 83% when it is operating at low power
density whereby pure oxygen and hydrogen
are used as reactants This is much more high
than that of world energy council having an
efficiency of 58% percent
Lead acid battery has a desired voltage and
current that ranges between 10 to 30 % of the
rated capacity. For instance when one takes a
10 Ah lead acid battery, it will charge at about
3A where lower percentages can be as well
recorded. Therefore considering lead acid
battery, a 10% charge rate is at 0.1 coulombs.
Difficulty of
disposing of the
power sources
The waste products obtained from the use of
hydrogen fuels cell is easily disposed and
used as environmental friendly products. For
example, Carbon (IV) oxide and water are the
main products. The methane is used together
with steam/water produced to form hydrogen
through a process of steam methane
reforming. Hence we can conclude that the
products can be disposed freely and also be
recycled.
Lead-acid batteries are a bulk of lead and
sulphuric acid, which is very corrosive.
Lead on the other side is a known toxic metal
having adverse effect on young lives such
causing of impairments on hearing, damage on
kidney and brain and other health associated
problems (Durr, Cruden, Gair, & McDonald,
2010)
On other hand the lead as by product is useful
in manufacturing of automobile having over 12
kilograms while 96% of lead is used in making
of lead acid battery.
Lead is exceedingly venomous metal and once
the battery creates blemished, it is important to
guarantee its appropriate accumulation and
eco-accommodating reusing. A solitary lead
acid battery discarded erroneously into a city
waste system, and not expelled before entering
an asset recuperation office for blended MSW,
could taint 25 tons of MSW and keep the
recuperation of the natural resources inside this
waste due to high lead level. (Jackey, 2014)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

EXTENDED RESPONSE TASK
References
Barsali, S., & Ceraolo, M. (2012). Dynamical Models of Lead-Acid Batteries:
Implementation Issues. IEEE Power Engineering Review , 1-3.
Cheung, T., Cheng, K., & Chan, H. (2009). Maintenance techniques for rechargeable battery
using pulse charging. London: New Jersey.
Durr, M., Cruden, A., Gair, S., & McDonald, J. (2010). Dynamic model of a lead acid battery
for use in a domestic fuel cell system. Journal of Power Source, 5-8.
Ferrari,, M., Sánchez, D., & Damo, U. (2017). Hybrid Systems Based on Solid Oxide Fuel
Cells: Modelling and Design. Manchester, UK.: John Wiley & Sons,.
Jackey, R. (2014). A simple, effective lead-acid battery modeling process for electrical
system component selection. SAE Technical Paper, 3.
Manwell, J., & McGowan, J. (2013). Lead acid battery storage model for hybrid energy
systems. Solar Energy, 2-5.
References
Barsali, S., & Ceraolo, M. (2012). Dynamical Models of Lead-Acid Batteries:
Implementation Issues. IEEE Power Engineering Review , 1-3.
Cheung, T., Cheng, K., & Chan, H. (2009). Maintenance techniques for rechargeable battery
using pulse charging. London: New Jersey.
Durr, M., Cruden, A., Gair, S., & McDonald, J. (2010). Dynamic model of a lead acid battery
for use in a domestic fuel cell system. Journal of Power Source, 5-8.
Ferrari,, M., Sánchez, D., & Damo, U. (2017). Hybrid Systems Based on Solid Oxide Fuel
Cells: Modelling and Design. Manchester, UK.: John Wiley & Sons,.
Jackey, R. (2014). A simple, effective lead-acid battery modeling process for electrical
system component selection. SAE Technical Paper, 3.
Manwell, J., & McGowan, J. (2013). Lead acid battery storage model for hybrid energy
systems. Solar Energy, 2-5.
1 out of 4
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.