Strategies for Lead Removal from Potable Water: A Comprehensive Report
VerifiedAdded on 2020/03/28
|25
|8451
|67
Report
AI Summary
This report delves into the critical issue of lead contamination in potable water, a significant global health concern. It begins by outlining how lead enters water supplies, often through corroded pipes and fixtures. The report then details the adverse health effects of lead exposure, particularly on children, including neurological damage and developmental delays. A substantial portion of the report is dedicated to exploring various lead removal methods, both conventional and advanced. Conventional methods discussed include distillation, reverse osmosis, activated carbon filtration, and ion exchange, while advanced methods encompass alkali ash material permeable reactive barriers, electrocoagulation, ultrafiltration, electrodialysis, and the use of nanomaterials. The report also considers factors influencing the selection of a water treatment method. Overall, the report underscores the importance of ensuring lead-free potable water to protect public health, offering a comprehensive overview of the problem and its potential solutions.

Removal of Lead from Potable Water 1
REMOVAL OF LEAD FROM POTABLE WATER
Name
Course
Professor
University
City/state
Date
REMOVAL OF LEAD FROM POTABLE WATER
Name
Course
Professor
University
City/state
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Removal of Lead from Potable Water 2
Table of Contents
1. Introduction.......................................................................................................................................3
2. How Lead Gets into Potable Water..................................................................................................4
3. Health impacts of lead contaminations............................................................................................5
4. Methods of removing lead from potable water................................................................................6
4.1. Conventional lead removal methods........................................................................................6
4.1.1. Distillation...........................................................................................................................6
4.1.2. Reverse osmosis...................................................................................................................7
4.1.3. Activated carbon filtration.................................................................................................10
4.1.4. Ion exchange......................................................................................................................12
4.2. Advanced lead removal methods............................................................................................14
4.2.1. Alkali Ash Material Permeable Reactive Barrier (AAM-PRB)..........................................14
4.2.2. Electrocoagulation.............................................................................................................15
4.2.3. Ultrafiltration (UF) system.................................................................................................15
4.2.4. Electrodialysis (ED)...........................................................................................................16
4.2.5. Nanomaterials and nanoparticles.......................................................................................16
5. Factors to consider when selecting a water treatment method....................................................17
6. Conclusion........................................................................................................................................18
References................................................................................................................................................19
Table of Contents
1. Introduction.......................................................................................................................................3
2. How Lead Gets into Potable Water..................................................................................................4
3. Health impacts of lead contaminations............................................................................................5
4. Methods of removing lead from potable water................................................................................6
4.1. Conventional lead removal methods........................................................................................6
4.1.1. Distillation...........................................................................................................................6
4.1.2. Reverse osmosis...................................................................................................................7
4.1.3. Activated carbon filtration.................................................................................................10
4.1.4. Ion exchange......................................................................................................................12
4.2. Advanced lead removal methods............................................................................................14
4.2.1. Alkali Ash Material Permeable Reactive Barrier (AAM-PRB)..........................................14
4.2.2. Electrocoagulation.............................................................................................................15
4.2.3. Ultrafiltration (UF) system.................................................................................................15
4.2.4. Electrodialysis (ED)...........................................................................................................16
4.2.5. Nanomaterials and nanoparticles.......................................................................................16
5. Factors to consider when selecting a water treatment method....................................................17
6. Conclusion........................................................................................................................................18
References................................................................................................................................................19

Removal of Lead from Potable Water 3
1. Introduction
Water is a very valuable natural resource to human beings. The water covers about 71% of
the earth’s surface (Perlman, 2016) but only about 0.03% of this water is usable by humans
(Mullen, 2012). The water is also vulnerable to numerous sources of contamination, both natural
and manmade. This has resulted to global water crisis (Harhay, 2011) because millions of people
across the world do not have access to safe potable water. Today, inadequate or limited supply of
fresh water has become one of the most pressing global issues (Srinivasan, et al., 2012). Each
year, about half a billion people across the world are affected by severe water scarcity
(Mekonnen & Hoekstra, 2016). Some of these people end up using contaminated water, which is
unsafe for drinking. Contaminated water is very hazardous to human life as it can transmit
diseases like cholera, diarrhea, typhoid, dysentery, etc. It is estimated that contaminated potable
water causes more than half a million diarrheal deaths every year (World Health Organization,
2017). This number is expected to rise with the rapidly growing global population that is
estimated to reach 9.8 billion by 2050 (United Nations, 2017). Lead is one of the major toxic
inorganic chemicals that contaminate water in many parts of the world. This compound is
dangerous both to the ecosystem and human health (Verstraeten, et al., 2008). The problem of
lead contamination in potable water is not new as this heavy metal started being used in making
water supply pipes and plumbing fixtures many years ago (Payne, 2008). Lead is a corrosion-
resistant soft material that is mainly used to making pipe lines, brass fixtures and lead solders.
The metal can also be added other metal alloys like bronze and brass, hence it is used for making
water fixtures, fittings and faucets. Lead is a harmful metal to health of both children and adults,
with children being the most affected. This makes lead contamination in potable water a major
health problem across the world.
Lead contamination in potable water has been reported in different countries, including the
U.S., Canada, Australia, Europe, United Kingdom and China, among others. Different countries
have formulated varied approaches of reducing lead contamination in potable water. Some of
these include prohibiting use of lead pipes in water supply systems and lead-based solders,
plumbing fixtures, fittings or faucets and setting maximum acceptable concentration of lead in
potable water. The maximum acceptable concentration of lead in potable water that has been set
by the World Health Organization (WHO) is 0.01 mg/L or 10 μg/L (Lead Free Water, 2015).
This is the water quality standard that is used in many countries including Australia, Canada,
1. Introduction
Water is a very valuable natural resource to human beings. The water covers about 71% of
the earth’s surface (Perlman, 2016) but only about 0.03% of this water is usable by humans
(Mullen, 2012). The water is also vulnerable to numerous sources of contamination, both natural
and manmade. This has resulted to global water crisis (Harhay, 2011) because millions of people
across the world do not have access to safe potable water. Today, inadequate or limited supply of
fresh water has become one of the most pressing global issues (Srinivasan, et al., 2012). Each
year, about half a billion people across the world are affected by severe water scarcity
(Mekonnen & Hoekstra, 2016). Some of these people end up using contaminated water, which is
unsafe for drinking. Contaminated water is very hazardous to human life as it can transmit
diseases like cholera, diarrhea, typhoid, dysentery, etc. It is estimated that contaminated potable
water causes more than half a million diarrheal deaths every year (World Health Organization,
2017). This number is expected to rise with the rapidly growing global population that is
estimated to reach 9.8 billion by 2050 (United Nations, 2017). Lead is one of the major toxic
inorganic chemicals that contaminate water in many parts of the world. This compound is
dangerous both to the ecosystem and human health (Verstraeten, et al., 2008). The problem of
lead contamination in potable water is not new as this heavy metal started being used in making
water supply pipes and plumbing fixtures many years ago (Payne, 2008). Lead is a corrosion-
resistant soft material that is mainly used to making pipe lines, brass fixtures and lead solders.
The metal can also be added other metal alloys like bronze and brass, hence it is used for making
water fixtures, fittings and faucets. Lead is a harmful metal to health of both children and adults,
with children being the most affected. This makes lead contamination in potable water a major
health problem across the world.
Lead contamination in potable water has been reported in different countries, including the
U.S., Canada, Australia, Europe, United Kingdom and China, among others. Different countries
have formulated varied approaches of reducing lead contamination in potable water. Some of
these include prohibiting use of lead pipes in water supply systems and lead-based solders,
plumbing fixtures, fittings or faucets and setting maximum acceptable concentration of lead in
potable water. The maximum acceptable concentration of lead in potable water that has been set
by the World Health Organization (WHO) is 0.01 mg/L or 10 μg/L (Lead Free Water, 2015).
This is the water quality standard that is used in many countries including Australia, Canada,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Removal of Lead from Potable Water 4
China, European Union and United Kingdom. The U.S. Environmental Protection Agency has
set the maximum acceptable concentration of lead in potable water to 15 μg/L or 0.015 mg/L
(U.S. Environmental Protection Agency, 2017). In these countries, water supply companies or
other relevant utilities are required to test water and ensure that the lead levels do not exceed the
maximum acceptable concentration. Cumulative exposure of lead can result to permanent
damage of important body organs such as the kidney, brain, heart, lungs and nervous system, or
even death.
The main reason why lead contamination is of great concern worldwide is because lead is
one the most toxic heavy metals. Lead exposure beyond recommended limits has several health
problems. For this reason, it is always important to ensure that potable water supplied to people
is free from lead. This report aims at investing sources of lead contamination in potable water,
health impacts of lead-contaminated water and various methods of removing lead from potable
water.
2. How Lead Gets into Potable Water
There are various ways in which lead gets into potable water. One of these ways is corrosion
of water faucets and fixtures (Zietz, et al., 2010), especially older ones that were mainly made of
lead. Most of water fixtures contain lead and when they get old, they can be easily corroded
(Torrice, 2016). When this happens, lead compound mixes with water thus contaminating it.
Solder and fittings used for connecting water supply pipes are other sources of lead in potable
water. With time, the solder and fittings may start corroding and the deposits get released into
potable water thus causing contamination. Lead water pipes are another source of lead
contamination in potable water. This was a major source of potable water contamination many
years back because most of the water pipes and other plumbing fixtures were made from lead
(Rabin, 2008). This metal was preferred because it is easily malleable and stable. Today, use of
lead water pipes has been prohibited in many parts of the world thus reducing potable water
contamination resulting from lead plumbing pipes. Therefore it is apparent that potable water is
usually contaminated after leaving the treatment plant (Renner, 2010). This makes it quite
challenging to deal with lead contamination in potable water especially in areas that are still
using water supply systems built many years ago, which contain lead plumbing components.
China, European Union and United Kingdom. The U.S. Environmental Protection Agency has
set the maximum acceptable concentration of lead in potable water to 15 μg/L or 0.015 mg/L
(U.S. Environmental Protection Agency, 2017). In these countries, water supply companies or
other relevant utilities are required to test water and ensure that the lead levels do not exceed the
maximum acceptable concentration. Cumulative exposure of lead can result to permanent
damage of important body organs such as the kidney, brain, heart, lungs and nervous system, or
even death.
The main reason why lead contamination is of great concern worldwide is because lead is
one the most toxic heavy metals. Lead exposure beyond recommended limits has several health
problems. For this reason, it is always important to ensure that potable water supplied to people
is free from lead. This report aims at investing sources of lead contamination in potable water,
health impacts of lead-contaminated water and various methods of removing lead from potable
water.
2. How Lead Gets into Potable Water
There are various ways in which lead gets into potable water. One of these ways is corrosion
of water faucets and fixtures (Zietz, et al., 2010), especially older ones that were mainly made of
lead. Most of water fixtures contain lead and when they get old, they can be easily corroded
(Torrice, 2016). When this happens, lead compound mixes with water thus contaminating it.
Solder and fittings used for connecting water supply pipes are other sources of lead in potable
water. With time, the solder and fittings may start corroding and the deposits get released into
potable water thus causing contamination. Lead water pipes are another source of lead
contamination in potable water. This was a major source of potable water contamination many
years back because most of the water pipes and other plumbing fixtures were made from lead
(Rabin, 2008). This metal was preferred because it is easily malleable and stable. Today, use of
lead water pipes has been prohibited in many parts of the world thus reducing potable water
contamination resulting from lead plumbing pipes. Therefore it is apparent that potable water is
usually contaminated after leaving the treatment plant (Renner, 2010). This makes it quite
challenging to deal with lead contamination in potable water especially in areas that are still
using water supply systems built many years ago, which contain lead plumbing components.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Removal of Lead from Potable Water 5
Lead may also find its way into potable water through pollution of natural water sources such
as rivers, lakes, oceans, etc. This can be through direct disposal of wastes containing lead into the
water source, infiltration from a landfill or weathering of rocks containing lead into groundwater
(Federal-Provincial-Territorial Committee on Drinking Water, 2016). In general, lead
contamination is potable water is primarily caused by wearing away or corrosion of lead-
containing materials in plumbing and water distribution systems. These materials comprise of
lead pipes, lead-based solders (Nguyen, et al., 2010) that are used for joining copper pipes, and
chrome plated and brass faucets, valves and fittings (Department of Public Health, (n.d.)). A very
small percentage of lead contamination in potable water is caused by pollution of natural sources
(World Health Organization, 2011).
There are also several factors that determine the extent to which potable water gets
contaminated with lead. These factors include: water chemistry (i.e. alkalinity and acidity) and
amount and types of minerals present in the water; water temperature; amount of lead coming in
contact with potable water; amount of water pipe corrosion or wear; the time the water stays in
supply pipes; water corrosivity (Swistock, 2017); and the presence of coatings or protective
scales in plumbing pipes, fixtures, fittings and faucets (United States Environmental Protection
Agency, 2017).
3. Health impacts of lead contaminations
Lean contamination in potable water causes a wide range of health problems in infants,
children and adults. Lead, which is a toxic metal, affects several body systems, including
cardiovascular, hematologic, neurologic, renal and gastrointestinal systems. The most common
problems in infants and children are delayed neurologic, mental or physical development
(Renner, 2009), anemia, hearing problems, slowed growth, brain damage, headaches, mood
disorders, hyperactivity, pica (easting strange stuffs such as paint chips, dirt, etc.) and lower IQ
(reduced intelligence) and learning and behavior problems. Affected children usually show
deficits in learning and attention span abilities. In adults, common health problems resulting
from lead contamination in potable water include: kidney problems, heart diseases (Navas-
Acien, et al., 2007), high blood pressure, hypertension, cancer, reproductive problems
(infertility) (Woodruff, et al., 2008) joint and muscle pain, digestive problems, loss of appetite,
(infertility) (Brown & Margolis, 2012), mood disorders, blood disorders (Iqbal, et al., 2008),
Lead may also find its way into potable water through pollution of natural water sources such
as rivers, lakes, oceans, etc. This can be through direct disposal of wastes containing lead into the
water source, infiltration from a landfill or weathering of rocks containing lead into groundwater
(Federal-Provincial-Territorial Committee on Drinking Water, 2016). In general, lead
contamination is potable water is primarily caused by wearing away or corrosion of lead-
containing materials in plumbing and water distribution systems. These materials comprise of
lead pipes, lead-based solders (Nguyen, et al., 2010) that are used for joining copper pipes, and
chrome plated and brass faucets, valves and fittings (Department of Public Health, (n.d.)). A very
small percentage of lead contamination in potable water is caused by pollution of natural sources
(World Health Organization, 2011).
There are also several factors that determine the extent to which potable water gets
contaminated with lead. These factors include: water chemistry (i.e. alkalinity and acidity) and
amount and types of minerals present in the water; water temperature; amount of lead coming in
contact with potable water; amount of water pipe corrosion or wear; the time the water stays in
supply pipes; water corrosivity (Swistock, 2017); and the presence of coatings or protective
scales in plumbing pipes, fixtures, fittings and faucets (United States Environmental Protection
Agency, 2017).
3. Health impacts of lead contaminations
Lean contamination in potable water causes a wide range of health problems in infants,
children and adults. Lead, which is a toxic metal, affects several body systems, including
cardiovascular, hematologic, neurologic, renal and gastrointestinal systems. The most common
problems in infants and children are delayed neurologic, mental or physical development
(Renner, 2009), anemia, hearing problems, slowed growth, brain damage, headaches, mood
disorders, hyperactivity, pica (easting strange stuffs such as paint chips, dirt, etc.) and lower IQ
(reduced intelligence) and learning and behavior problems. Affected children usually show
deficits in learning and attention span abilities. In adults, common health problems resulting
from lead contamination in potable water include: kidney problems, heart diseases (Navas-
Acien, et al., 2007), high blood pressure, hypertension, cancer, reproductive problems
(infertility) (Woodruff, et al., 2008) joint and muscle pain, digestive problems, loss of appetite,
(infertility) (Brown & Margolis, 2012), mood disorders, blood disorders (Iqbal, et al., 2008),

Removal of Lead from Potable Water 6
Alzheimer disease, and autoimmune diseases such as multiple sclerosis, lupus, fibromyalgia,
chronic fatigue syndrome, rheumatoid arthritis, type 1 diabetes, etc. (Hayward, 2017). It also
affects pregnant women and their unborn babies. These effects include: premature birth, low
birth weight, gestational hypertension, miscarriage or abortion and reduced fetus growth.
Lactating mothers can also transmit lead to their babies through breast milk (United States
Environmental Protection Agency, 2017). Pregnant women, fetuses, infants and children are
more vulnerable to potable water contamination by lead because they have sensitive tissues
capable of absorbing lead more easily. When exposed to same level of lead, children and adults
will absorb 30-75% and 11% of the lead respectively (Water Quality Association, 2016).
Therefore lead is a major threat to public health and there is need for more people to have access
to information so that they can establish ways of preventing these effects. If not treated properly
or managed on time, lead exposure through potable water can lead to death.
4. Methods of removing lead from potable water
There are different methods of removing lead from potable water. Some of these methods are
discussed below.
4.1. Conventional lead removal methods
4.1.1. Distillation
Distillation is a conventional and effective method of treating water for domestic and
commercial use. This method has been in use for millennia. The method removes a wide range of
impurities from water, including bacteria, dissolved solids, nitrates, hardness, lead, sodium, etc.
The principle of water distillation has remained the same over the years, only the type and layout
of equipment used keeps on changing. The process starts with boiling water in a container. The
steam produced is then directed into cooling tubes where it is condensed then collected in a
second container as purified water. In this process, almost all impurities present in water are left
in the first container.
Distillation is suitable for removing chemicals or contaminants whose boiling point is
greater than that of water. The boiling point of water and lead is 100 °C and 1,749 °C
respectively. When lead-contaminated water is heated, the water starts evaporating when
temperature reaches 100 °C. At this point, the lead is still very away from reaching its boiling
point. Therefore the water evaporates and the escaping steam is collected and directed into the
Alzheimer disease, and autoimmune diseases such as multiple sclerosis, lupus, fibromyalgia,
chronic fatigue syndrome, rheumatoid arthritis, type 1 diabetes, etc. (Hayward, 2017). It also
affects pregnant women and their unborn babies. These effects include: premature birth, low
birth weight, gestational hypertension, miscarriage or abortion and reduced fetus growth.
Lactating mothers can also transmit lead to their babies through breast milk (United States
Environmental Protection Agency, 2017). Pregnant women, fetuses, infants and children are
more vulnerable to potable water contamination by lead because they have sensitive tissues
capable of absorbing lead more easily. When exposed to same level of lead, children and adults
will absorb 30-75% and 11% of the lead respectively (Water Quality Association, 2016).
Therefore lead is a major threat to public health and there is need for more people to have access
to information so that they can establish ways of preventing these effects. If not treated properly
or managed on time, lead exposure through potable water can lead to death.
4. Methods of removing lead from potable water
There are different methods of removing lead from potable water. Some of these methods are
discussed below.
4.1. Conventional lead removal methods
4.1.1. Distillation
Distillation is a conventional and effective method of treating water for domestic and
commercial use. This method has been in use for millennia. The method removes a wide range of
impurities from water, including bacteria, dissolved solids, nitrates, hardness, lead, sodium, etc.
The principle of water distillation has remained the same over the years, only the type and layout
of equipment used keeps on changing. The process starts with boiling water in a container. The
steam produced is then directed into cooling tubes where it is condensed then collected in a
second container as purified water. In this process, almost all impurities present in water are left
in the first container.
Distillation is suitable for removing chemicals or contaminants whose boiling point is
greater than that of water. The boiling point of water and lead is 100 °C and 1,749 °C
respectively. When lead-contaminated water is heated, the water starts evaporating when
temperature reaches 100 °C. At this point, the lead is still very away from reaching its boiling
point. Therefore the water evaporates and the escaping steam is collected and directed into the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Removal of Lead from Potable Water 7
cooling tubes, leaving lead and other impurities in the original boiling container. The cooling
tubes deposit the steam into a condenser where the steam condenses back to liquid form. This
liquid is very pure and suitable for drinking.
4.1.1.1. Advantages
Pure water: distillation is a very efficient method of removing contaminants with boiling
point greater than that of water. This makes it most suitable for removing lead from drinking
water. Besides heavy metals, distillation also removes all pathogens.
Reliability: the quality and purity of water obtained from distillation process always
remains high as long as the distillation unit is working properly and kept clean.
Reusability: distillation method can be reused over and over again without replacing any
component.
4.1.1.2. Disadvantages
Wasteful: this process wastes a lot of water as only about 20% of the water gets purified
while the remaining 80% is discarded with other contaminants.
Mineral-free water: the water obtained from distillation process is mineral-free and
tasteless because all minerals, including beneficial minerals, are removed. The mineral-free
water is also more acidic making it dangerous to the human body.
Not suitable for contaminants with low boiling point: any contaminants whose boiling
point is lower than that of water will find its way in the water collected in the final container.
Therefore distilled water will still contain all contaminants with boiling point lower than that of
water.
Energy: this method requires substantial amount of power for heating the water. Some
water distillation units have been designed to use renewable energy such as solar power so as to
reduce energy bills (Gugulothu, et al., 2015).
Slow: distillation method is relatively slow because the water output is very low.
4.1.2. Reverse osmosis
This is one of the most common and effective methods used to remove lead from potable
water for domestic, commercial and industrial uses because its efficiency is about 98%. Besides
cooling tubes, leaving lead and other impurities in the original boiling container. The cooling
tubes deposit the steam into a condenser where the steam condenses back to liquid form. This
liquid is very pure and suitable for drinking.
4.1.1.1. Advantages
Pure water: distillation is a very efficient method of removing contaminants with boiling
point greater than that of water. This makes it most suitable for removing lead from drinking
water. Besides heavy metals, distillation also removes all pathogens.
Reliability: the quality and purity of water obtained from distillation process always
remains high as long as the distillation unit is working properly and kept clean.
Reusability: distillation method can be reused over and over again without replacing any
component.
4.1.1.2. Disadvantages
Wasteful: this process wastes a lot of water as only about 20% of the water gets purified
while the remaining 80% is discarded with other contaminants.
Mineral-free water: the water obtained from distillation process is mineral-free and
tasteless because all minerals, including beneficial minerals, are removed. The mineral-free
water is also more acidic making it dangerous to the human body.
Not suitable for contaminants with low boiling point: any contaminants whose boiling
point is lower than that of water will find its way in the water collected in the final container.
Therefore distilled water will still contain all contaminants with boiling point lower than that of
water.
Energy: this method requires substantial amount of power for heating the water. Some
water distillation units have been designed to use renewable energy such as solar power so as to
reduce energy bills (Gugulothu, et al., 2015).
Slow: distillation method is relatively slow because the water output is very low.
4.1.2. Reverse osmosis
This is one of the most common and effective methods used to remove lead from potable
water for domestic, commercial and industrial uses because its efficiency is about 98%. Besides
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Removal of Lead from Potable Water 8
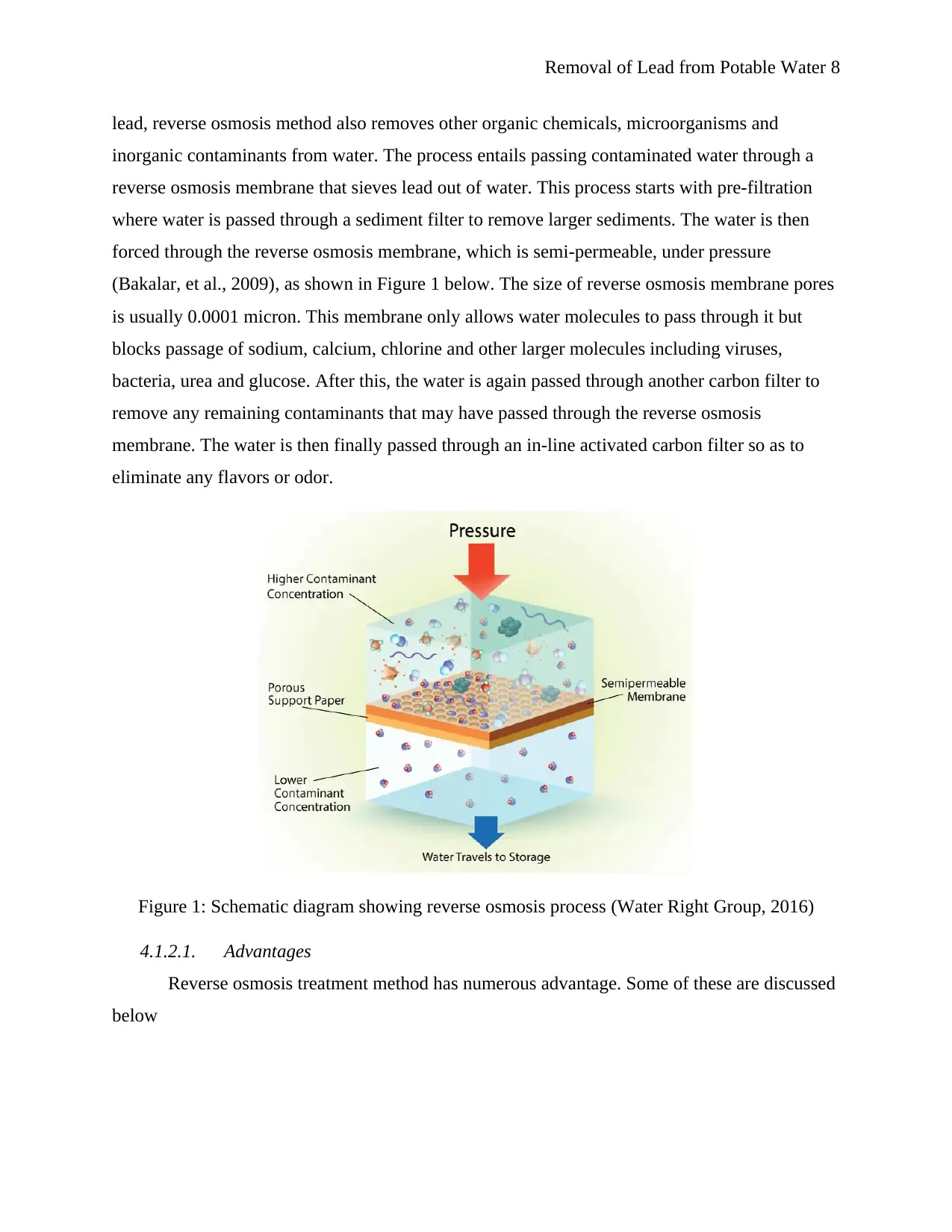
lead, reverse osmosis method also removes other organic chemicals, microorganisms and
inorganic contaminants from water. The process entails passing contaminated water through a
reverse osmosis membrane that sieves lead out of water. This process starts with pre-filtration
where water is passed through a sediment filter to remove larger sediments. The water is then
forced through the reverse osmosis membrane, which is semi-permeable, under pressure
(Bakalar, et al., 2009), as shown in Figure 1 below. The size of reverse osmosis membrane pores
is usually 0.0001 micron. This membrane only allows water molecules to pass through it but
blocks passage of sodium, calcium, chlorine and other larger molecules including viruses,
bacteria, urea and glucose. After this, the water is again passed through another carbon filter to
remove any remaining contaminants that may have passed through the reverse osmosis
membrane. The water is then finally passed through an in-line activated carbon filter so as to
eliminate any flavors or odor.
Figure 1: Schematic diagram showing reverse osmosis process (Water Right Group, 2016)
4.1.2.1. Advantages
Reverse osmosis treatment method has numerous advantage. Some of these are discussed
below
lead, reverse osmosis method also removes other organic chemicals, microorganisms and
inorganic contaminants from water. The process entails passing contaminated water through a
reverse osmosis membrane that sieves lead out of water. This process starts with pre-filtration
where water is passed through a sediment filter to remove larger sediments. The water is then
forced through the reverse osmosis membrane, which is semi-permeable, under pressure
(Bakalar, et al., 2009), as shown in Figure 1 below. The size of reverse osmosis membrane pores
is usually 0.0001 micron. This membrane only allows water molecules to pass through it but
blocks passage of sodium, calcium, chlorine and other larger molecules including viruses,
bacteria, urea and glucose. After this, the water is again passed through another carbon filter to
remove any remaining contaminants that may have passed through the reverse osmosis
membrane. The water is then finally passed through an in-line activated carbon filter so as to
eliminate any flavors or odor.
Figure 1: Schematic diagram showing reverse osmosis process (Water Right Group, 2016)
4.1.2.1. Advantages
Reverse osmosis treatment method has numerous advantage. Some of these are discussed
below

Removal of Lead from Potable Water 9
Removes contaminants: reverse osmosis methods removes almost 100% of contaminants
from water. This includes lead, arsenic, copper, sodium, nitrates, organic chemicals, viruses,
protozoa, bacteria, etc.
Cost-effective: this method produces very clean potable water for about $0.25 per day
(Linton, 2016), making it affordable even for domestic uses.
Environmental friendly: the method does not require uses of large amount of energy that
could otherwise result to emission of greenhouse gases to the environment and it does not use or
produce any toxic chemicals. Additionally, it eliminates the need of buying water bottles that
could end up being disposed in landfills because it provides clean potable water at all times.
Reduces odor and improves taste: reverse osmosis not only removes lead but numerous
other contaminants including arsenic, nitrates, nickel, chlorine and total dissolved solids.
Removal of these impurities leaves clean water that tastes better and has no odor.
Small size: reverse osmosis systems are usually small in size thus suitable for home uses.
By removing most of the metals and dissolved minerals from water, reverse osmosis
helps in preventing corrosion of plumbing systems.
4.1.2.2. Disadvantages
This method removes all chemical materials and minerals from water, including
beneficial and/or healthy minerals such as manganese and iron.
By removing most of the minerals, this method leaves the water more acidic.
The method requires large amount of water because only about 5-15% of the water that
gets pushed through the reverse osmosis system returns (Craig, (n.d.)). The remaining water
leaves the system as wastewater.
The method is consuming meaning it requires a lot of time in order to treat a substantial
amount of water. For example, filtering one gallon of water using reverse osmosis system may
take between 3 and 4 hours.
Reverse osmosis systems usually require frequent maintenance so as to maintain high
level of effectiveness in removing lead from the water.
Removes contaminants: reverse osmosis methods removes almost 100% of contaminants
from water. This includes lead, arsenic, copper, sodium, nitrates, organic chemicals, viruses,
protozoa, bacteria, etc.
Cost-effective: this method produces very clean potable water for about $0.25 per day
(Linton, 2016), making it affordable even for domestic uses.
Environmental friendly: the method does not require uses of large amount of energy that
could otherwise result to emission of greenhouse gases to the environment and it does not use or
produce any toxic chemicals. Additionally, it eliminates the need of buying water bottles that
could end up being disposed in landfills because it provides clean potable water at all times.
Reduces odor and improves taste: reverse osmosis not only removes lead but numerous
other contaminants including arsenic, nitrates, nickel, chlorine and total dissolved solids.
Removal of these impurities leaves clean water that tastes better and has no odor.
Small size: reverse osmosis systems are usually small in size thus suitable for home uses.
By removing most of the metals and dissolved minerals from water, reverse osmosis
helps in preventing corrosion of plumbing systems.
4.1.2.2. Disadvantages
This method removes all chemical materials and minerals from water, including
beneficial and/or healthy minerals such as manganese and iron.
By removing most of the minerals, this method leaves the water more acidic.
The method requires large amount of water because only about 5-15% of the water that
gets pushed through the reverse osmosis system returns (Craig, (n.d.)). The remaining water
leaves the system as wastewater.
The method is consuming meaning it requires a lot of time in order to treat a substantial
amount of water. For example, filtering one gallon of water using reverse osmosis system may
take between 3 and 4 hours.
Reverse osmosis systems usually require frequent maintenance so as to maintain high
level of effectiveness in removing lead from the water.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Removal of Lead from Potable Water 10
4.1.3. Activated carbon filtration
This is one of the oldest and common methods of water treatment (Bhatnagar, et al.,
2013). The method is also known as adsorption. Most of the activated carbon filters used today
have been improved and therefore are more effective in water treatment than those used several
years back (Egbon, et al., 2013). Activated carbon filtration method removes lead from potable
water using a special type of carbon filter. The lead and other contaminants get removed from
water through a process known as adsorption. Activated carbon filtration system uses reactive or
adsorptive filters. These filters contain a medium that reacts with or adsorbs the lead present in
water. The medium can be made from petroleum coke, wood products, lignite, peanut shells,
bituminous coal or coconut shell. When the water is passed through the activated carbon filter, it
gets attracted and adsorbed or held on the carbon particles surface. The adsorption process
efficiency depends on carbon characteristics (pore size, particle size, hardness, density and
surface area) and contaminant characteristics (solubility, attraction to carbon surface and
concentration) (Dvorak & Skipton, 2013).
Before the start of water treatment process, the carbon medium has to be activated first.
This is done by subjecting it to high temperature of up to 1260 °C and steam in absence of
oxygen. Sometimes the carbon medium may be coated with a specific compound or processed
using acid wash so as to improve its capability of removing particular contaminants from water.
This activation process generates carbon that has numerous small pores thus its surface area is
also very high. After that, the carbon medium is crushed to create a pulverized or granular carbon
product creating smaller particles whose outside surface area is more available for adsorption of
contaminants.
Once the carbon medium or activated carbon filter has been prepared, water is passed
through it. Since the filter is made from carbon that is more effective in removing lead, the lead
particles present in water will get strongly and easily adsorbed onto the surface of the carbon
filter. The efficiency of lead adsorption is also directly proportional to the length of contact time
between carbon and water. When the same activated carbon filter is used for a long period of
time, it gets saturated. This basically means that its pores become filled with lead contaminant
and therefore further adsorption cannot take place. In such cases, the filters have to be cleaned or
replaced.
4.1.3. Activated carbon filtration
This is one of the oldest and common methods of water treatment (Bhatnagar, et al.,
2013). The method is also known as adsorption. Most of the activated carbon filters used today
have been improved and therefore are more effective in water treatment than those used several
years back (Egbon, et al., 2013). Activated carbon filtration method removes lead from potable
water using a special type of carbon filter. The lead and other contaminants get removed from
water through a process known as adsorption. Activated carbon filtration system uses reactive or
adsorptive filters. These filters contain a medium that reacts with or adsorbs the lead present in
water. The medium can be made from petroleum coke, wood products, lignite, peanut shells,
bituminous coal or coconut shell. When the water is passed through the activated carbon filter, it
gets attracted and adsorbed or held on the carbon particles surface. The adsorption process
efficiency depends on carbon characteristics (pore size, particle size, hardness, density and
surface area) and contaminant characteristics (solubility, attraction to carbon surface and
concentration) (Dvorak & Skipton, 2013).
Before the start of water treatment process, the carbon medium has to be activated first.
This is done by subjecting it to high temperature of up to 1260 °C and steam in absence of
oxygen. Sometimes the carbon medium may be coated with a specific compound or processed
using acid wash so as to improve its capability of removing particular contaminants from water.
This activation process generates carbon that has numerous small pores thus its surface area is
also very high. After that, the carbon medium is crushed to create a pulverized or granular carbon
product creating smaller particles whose outside surface area is more available for adsorption of
contaminants.
Once the carbon medium or activated carbon filter has been prepared, water is passed
through it. Since the filter is made from carbon that is more effective in removing lead, the lead
particles present in water will get strongly and easily adsorbed onto the surface of the carbon
filter. The efficiency of lead adsorption is also directly proportional to the length of contact time
between carbon and water. When the same activated carbon filter is used for a long period of
time, it gets saturated. This basically means that its pores become filled with lead contaminant
and therefore further adsorption cannot take place. In such cases, the filters have to be cleaned or
replaced.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Removal of Lead from Potable Water 11
4.1.3.1. Advantages
Installation and maintenance: it is very easy to install an activated carbon filtration
system and maintain it. These processes require less tools and equipment.
Low operating costs: the operation costs of activated carbon filtration method are
relatively low because they usually require less energy and maintenance needs are also low.
Efficiency: this method is very efficient in removing lead from water as long as the
activated carbon filter is made from carbon medium that has high affinity for lead.
Flexibility: the method can also be used to remove lead from water at point-of-use (such
as community or household filters) or at point-of-entry (such as wastewater treatment facilities or
semi-centralized potable water treatment facilities) (Mazille & Spuhler, 2011).
Availability: this method can be used anywhere because activated carbon filters can be
made in any part of the world. The required raw materials for making activated carbon are
abundant.
Reliability: this method is very reliable in removing lea from water as long as the
activated carbon filter is made or selected by considering the characteristics of the contaminant
(composition, solubility, quantity and affinity).
4.1.3.2. Disadvantages
Maintenance: activated carbon filters require regular regeneration or replacement. This is
because the filters, after adsorbing lead for some time, get clogged or saturated making them
ineffective in removing lead from the water. The filters can be replaced based on the
manufacturer’s recommendations or the user can calculate replacement intervals based on the
amount of lead being removed and quantity of water being purified by the system on daily basis.
Limited lifetime: activated carbon filters’ lifetime is limited. After these filters have been
used for a long time, their surfaces get saturated with lead or other water contaminants and they
stop purifying water. Sometimes backwashing the filter may not be enough leaving replacement
as the only best solution.
4.1.3.1. Advantages
Installation and maintenance: it is very easy to install an activated carbon filtration
system and maintain it. These processes require less tools and equipment.
Low operating costs: the operation costs of activated carbon filtration method are
relatively low because they usually require less energy and maintenance needs are also low.
Efficiency: this method is very efficient in removing lead from water as long as the
activated carbon filter is made from carbon medium that has high affinity for lead.
Flexibility: the method can also be used to remove lead from water at point-of-use (such
as community or household filters) or at point-of-entry (such as wastewater treatment facilities or
semi-centralized potable water treatment facilities) (Mazille & Spuhler, 2011).
Availability: this method can be used anywhere because activated carbon filters can be
made in any part of the world. The required raw materials for making activated carbon are
abundant.
Reliability: this method is very reliable in removing lea from water as long as the
activated carbon filter is made or selected by considering the characteristics of the contaminant
(composition, solubility, quantity and affinity).
4.1.3.2. Disadvantages
Maintenance: activated carbon filters require regular regeneration or replacement. This is
because the filters, after adsorbing lead for some time, get clogged or saturated making them
ineffective in removing lead from the water. The filters can be replaced based on the
manufacturer’s recommendations or the user can calculate replacement intervals based on the
amount of lead being removed and quantity of water being purified by the system on daily basis.
Limited lifetime: activated carbon filters’ lifetime is limited. After these filters have been
used for a long time, their surfaces get saturated with lead or other water contaminants and they
stop purifying water. Sometimes backwashing the filter may not be enough leaving replacement
as the only best solution.

Removal of Lead from Potable Water 12
Skilled labour: removing lead from potable water using active carbon filtration method
requires skilled labour, at least occasionally. The skilled labour is needed for monitoring removal
efficiency and/or performance of the activated carbon filtration unit.
Water analysis: for the activated carbon filtration method to remove lead effectively from
the water, water analysis has to be carried out comprehensively. This helps in determining the
most effective type of activated carbon for removal of lead. The water analysis process may
require more resources, including time, personnel and money.
Energy: activated carbon filtration units are usually driven by power and therefore energy
must be provided.
This method also only separates lead and other contaminants from the water but it does
not destroy them. As a result of this, the adsorbed contaminants, including lead, may still find
themselves back in water creating more health problems.
4.1.4. Ion exchange
This is a water treatment method that uses ion exchange resins to remove contaminants
from the water. Ion exchange resins are simply colored synthetic spherical micro-porous beads of
size ranging between 0.5mm and 1mm and have pores on their surfaces for trapping and
absorbing contaminants present in water. Ion exchangers are water-insoluble substances that
exchange some of their ions with similar charged ions that are present in the media with which
they come in contact with (Kumar & Jain, 2013). The method treats water through exchange of
ions between the water and resin. Ion exchange resins contain loosely held positive and negative
ions. The positively charged ions are known as cations while the negatively charged ions are
called anions. There are two main types of ion exchange resins: cation exchange resin and anion
exchange resin (New Hampshire Department of Environmental Services, 2009). A cation
exchange resin is a type of resin that has a positive charge and is suitable for treating water
containing a positive charge whereas an anion exchange resin is a type of resin that has a
negative charge and is suitable for treating water containing a negative charge. Cation exchange
resins comprise of polystyrene chains of divinylbenzene covalent bonds. A cation exchange resin
is denser than an anion exchange resin thus the former has a greater exchange capacity than the
latter. A cation exchange resins also needs less volume than an anion exchange resin of the same
Skilled labour: removing lead from potable water using active carbon filtration method
requires skilled labour, at least occasionally. The skilled labour is needed for monitoring removal
efficiency and/or performance of the activated carbon filtration unit.
Water analysis: for the activated carbon filtration method to remove lead effectively from
the water, water analysis has to be carried out comprehensively. This helps in determining the
most effective type of activated carbon for removal of lead. The water analysis process may
require more resources, including time, personnel and money.
Energy: activated carbon filtration units are usually driven by power and therefore energy
must be provided.
This method also only separates lead and other contaminants from the water but it does
not destroy them. As a result of this, the adsorbed contaminants, including lead, may still find
themselves back in water creating more health problems.
4.1.4. Ion exchange
This is a water treatment method that uses ion exchange resins to remove contaminants
from the water. Ion exchange resins are simply colored synthetic spherical micro-porous beads of
size ranging between 0.5mm and 1mm and have pores on their surfaces for trapping and
absorbing contaminants present in water. Ion exchangers are water-insoluble substances that
exchange some of their ions with similar charged ions that are present in the media with which
they come in contact with (Kumar & Jain, 2013). The method treats water through exchange of
ions between the water and resin. Ion exchange resins contain loosely held positive and negative
ions. The positively charged ions are known as cations while the negatively charged ions are
called anions. There are two main types of ion exchange resins: cation exchange resin and anion
exchange resin (New Hampshire Department of Environmental Services, 2009). A cation
exchange resin is a type of resin that has a positive charge and is suitable for treating water
containing a positive charge whereas an anion exchange resin is a type of resin that has a
negative charge and is suitable for treating water containing a negative charge. Cation exchange
resins comprise of polystyrene chains of divinylbenzene covalent bonds. A cation exchange resin
is denser than an anion exchange resin thus the former has a greater exchange capacity than the
latter. A cation exchange resins also needs less volume than an anion exchange resin of the same
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 25
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





