HI5015: Legal Aspects - AbbVie Inc. International Business Analysis
VerifiedAdded on 2024/07/22
|11
|2492
|193
Report
AI Summary
This report analyzes the legal aspects of AbbVie Inc., a biopharmaceutical company operating in over 170 nations. It discusses key Australian laws like the Corporations Act 2001, Patents Act 1990, Trademark Act 1995, Therapeutic Goods Act 1989, and Gene Technology Act 2000, highlighting the importance of compliance for smooth business operations. The report also examines international agreements and conventions, such as the Convention on Biological Diversity and the Pharmaceutical Inspection Convention, and agreements with companies like Inventiva. It emphasizes how these legal frameworks and collaborations are crucial for AbbVie's global operations and the development of new therapeutic goods.

HI5015: LEGAL ASPECTS OF INTERNATIONAL
BUSINESS AND ENTERPRISE
1
BUSINESS AND ENTERPRISE
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
Question 1........................................................................................................................................3
Question 2........................................................................................................................................4
Question 3........................................................................................................................................6
Reference:........................................................................................................................................9
2
Question 1........................................................................................................................................3
Question 2........................................................................................................................................4
Question 3........................................................................................................................................6
Reference:........................................................................................................................................9
2
Question 1
In this assignment, the company selected for describing the legal aspects of business in multiple
nations is AbbVie Inc. It is a company which operates in the biopharmaceutical and
biotechnology sector of the business in the global market and has a place of business in more
than 170 nations. This company is a manufacturer in the pharmaceutical sector and the
manufacturing of the medicinal products is based on researches (AbbVie, 2018). The
establishment of this company was done in 2013 by separating it with Abbott Laboratories.
Moreover, the company is listed on the stock exchange of New York on 2nd January 2013.
The company is headquartered at Lake Bluff, Illinois, United States and from such headquarters,
the operation of the company all over the globe is organised and managed. The company also
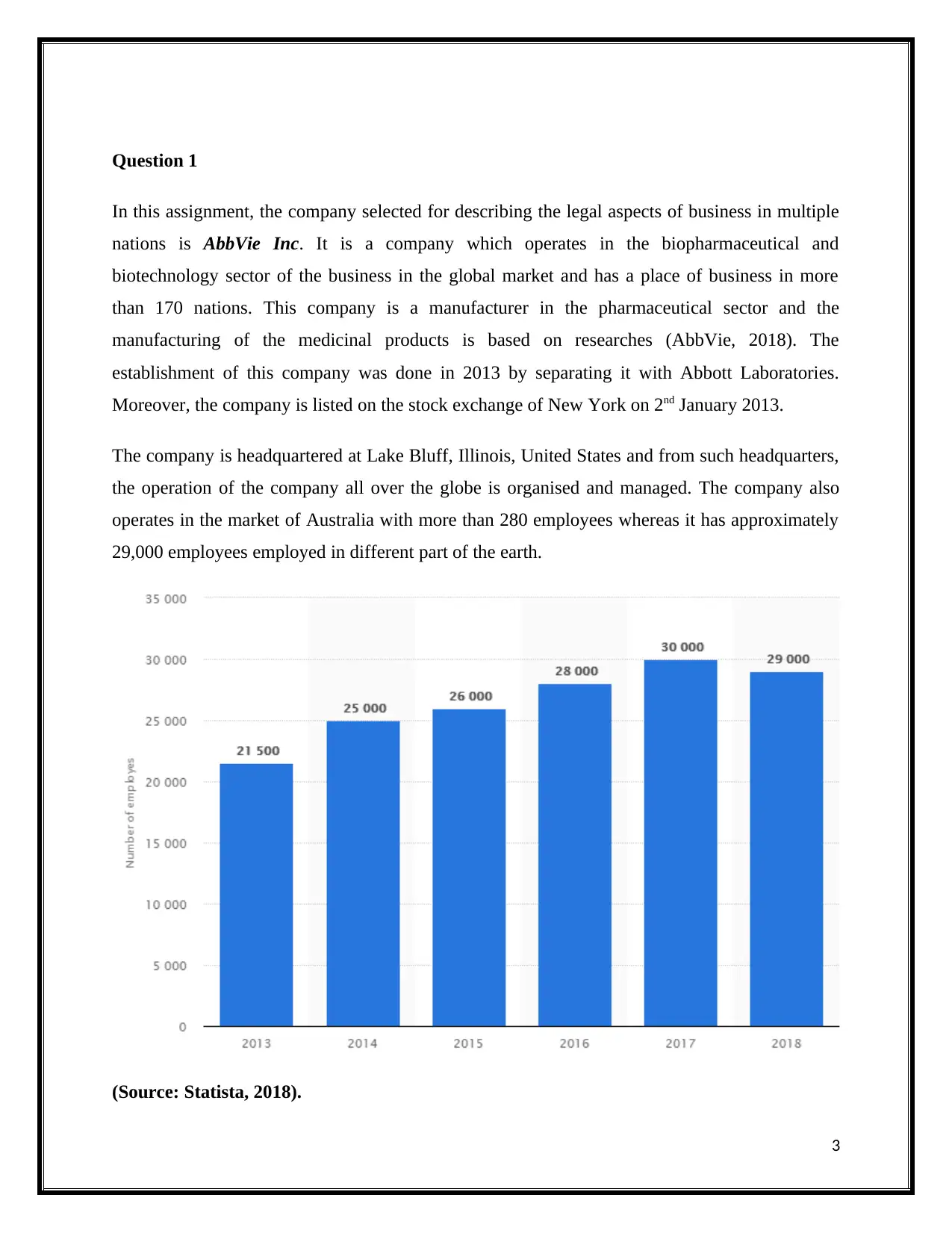
operates in the market of Australia with more than 280 employees whereas it has approximately
29,000 employees employed in different part of the earth.
(Source: Statista, 2018).
3
In this assignment, the company selected for describing the legal aspects of business in multiple
nations is AbbVie Inc. It is a company which operates in the biopharmaceutical and
biotechnology sector of the business in the global market and has a place of business in more
than 170 nations. This company is a manufacturer in the pharmaceutical sector and the
manufacturing of the medicinal products is based on researches (AbbVie, 2018). The
establishment of this company was done in 2013 by separating it with Abbott Laboratories.
Moreover, the company is listed on the stock exchange of New York on 2nd January 2013.
The company is headquartered at Lake Bluff, Illinois, United States and from such headquarters,
the operation of the company all over the globe is organised and managed. The company also
operates in the market of Australia with more than 280 employees whereas it has approximately
29,000 employees employed in different part of the earth.
(Source: Statista, 2018).
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Question 2
The company considered in this assignment, AbbVie Inc. provides the services of different
nations i.e. in more than 170 nations. Provisions of such services and conducting of business in
different nations attracts various laws or norms. In order to operate under the business
environment and the legal system of a number of nations, the requirements of the applicable laws
are to be met with by the personnel responsible for conducting the business of the company.
Compliance with the applicable requirements specified under the law provides better structuring
to the business activities of the company and encourages systematic conduction of such activities
in accordance with the laws. As per the legal system of Australia, certain laws are to be complied
with generally. AbbVie Inc. functions as a company in the business environment of Australia,
the foremost act for each and every company is the Corporations Act, 2001. This act is
considered as guidelines for every company as the management of every company from the time
of its establishment makes sure that all the requirements of the act is met with or satisfied. Even
at the time of company being wound up, the provisions of this act is to be satisfied. Australian
Securities and Investment Commission is an authority which is established to review and
monitor the functional and operating activities of the company. For conducting business, a
company is required to apply in the specified form to the authority mentioned above along which
legal fees. Once the company is registered with the Australian Securities and Investment
Commission then it can successfully run the business and provides its services. Moreover, from
the appointment of the directors, secretaries, managers, etc and until their removal or resignation,
all such activities are to be met with the provisions of this act. Non-compliance with the
provisions or the requirements of this act attracts fine under the law. Therefore, it is made sure
by the management of the pharmaceutical company, AbbVie Inc. that all the requirements of this
act are satisfied or met with in a fair and true manner.
As the company is a manufacturer of medicinal products which are based on research, it attracts
the provisions of the Patents Act 1990 and the Patents Regulations 1991. The company is
engaged in developing new drugs for the treatment of different diseases and this leads to the
illicit use of the newly developed drugs or the procedure by which it was developed by the
competitors of the developer company. Due to this, such competitors will be able to take
5
The company considered in this assignment, AbbVie Inc. provides the services of different
nations i.e. in more than 170 nations. Provisions of such services and conducting of business in
different nations attracts various laws or norms. In order to operate under the business
environment and the legal system of a number of nations, the requirements of the applicable laws
are to be met with by the personnel responsible for conducting the business of the company.
Compliance with the applicable requirements specified under the law provides better structuring
to the business activities of the company and encourages systematic conduction of such activities
in accordance with the laws. As per the legal system of Australia, certain laws are to be complied
with generally. AbbVie Inc. functions as a company in the business environment of Australia,
the foremost act for each and every company is the Corporations Act, 2001. This act is
considered as guidelines for every company as the management of every company from the time
of its establishment makes sure that all the requirements of the act is met with or satisfied. Even
at the time of company being wound up, the provisions of this act is to be satisfied. Australian
Securities and Investment Commission is an authority which is established to review and
monitor the functional and operating activities of the company. For conducting business, a
company is required to apply in the specified form to the authority mentioned above along which
legal fees. Once the company is registered with the Australian Securities and Investment
Commission then it can successfully run the business and provides its services. Moreover, from
the appointment of the directors, secretaries, managers, etc and until their removal or resignation,
all such activities are to be met with the provisions of this act. Non-compliance with the
provisions or the requirements of this act attracts fine under the law. Therefore, it is made sure
by the management of the pharmaceutical company, AbbVie Inc. that all the requirements of this
act are satisfied or met with in a fair and true manner.
As the company is a manufacturer of medicinal products which are based on research, it attracts
the provisions of the Patents Act 1990 and the Patents Regulations 1991. The company is
engaged in developing new drugs for the treatment of different diseases and this leads to the
illicit use of the newly developed drugs or the procedure by which it was developed by the
competitors of the developer company. Due to this, such competitors will be able to take
5

advantage of the products of the developer which is against the law. Therefore, it is necessary
that after the formulation of the product, a patent must be obtained by the developer company.
There are a series of steps which are to be followed by the management of the company for
getting patent for the new product. It is essential that for getting registered with the authority for
a patent in Australia, IP Australia, an application is to be made by the company seeking
protection under the act against the unlawful use of newly developed product or the procedure by
which such products are developed (Dutfield, 2017). There are two types of a patent which can
be applied with for protection to the products and procedures, these types are standard patent
which provides the protection in traditional form and other is innovation patent which is form
shorter period of time as it is unable to meet the requirement of a standard patent. Such patents
are valid for a period of 20 years whereas, for pharmaceutical substances, it is valid for a period
of 25 years (Moore, 2017). If after getting the patent rights, any other company makes use, sell,
make, import or holding the product for performing any of such actions then it will be considered
as an infringement of the patent rights which is punishable under the law. Such products or
procedures can be used for the purposes mentioned above such as making, selling, importing,
etc. only after consent of the patent holder has been obtained.
Another act, the provisions of which are to be followed by the company is the Trademark Act,
1995. As per the provisions of this act, protection is provided to the trademark of the company
against its use or use of the similar trademark by which the original product cannot be separated
from the other products. Trademark is the word, shape, color, symbol, mark, etc. which is
capable of distinguishing the products and services of one company from the rest of the
companies in the course of trade (Drahos, 2016). Trademarks can only be obtained by applying
to the authority of intellectual property i.e. IP Australia. However, in such case, certain
conditions are to be satisfied such the trademark must be different from the previously registered
trademark. Registration in the Trademark Act provides a right to the holder that only such holder
make use of the trademark, however, such right holder can also authorise other to make use of
such trademark (Gibson, 2017). It results in the infringement of a trademark, if the trademark
right holder is used by other company or person without prior consent of the holder.
Therapeutic Goods Act, 1989 is also to be followed by the company as provides permission for
the formulation of the therapeutic goods and in order to maintain the quality, safety, efficacy and
6
that after the formulation of the product, a patent must be obtained by the developer company.
There are a series of steps which are to be followed by the management of the company for
getting patent for the new product. It is essential that for getting registered with the authority for
a patent in Australia, IP Australia, an application is to be made by the company seeking
protection under the act against the unlawful use of newly developed product or the procedure by
which such products are developed (Dutfield, 2017). There are two types of a patent which can
be applied with for protection to the products and procedures, these types are standard patent
which provides the protection in traditional form and other is innovation patent which is form
shorter period of time as it is unable to meet the requirement of a standard patent. Such patents
are valid for a period of 20 years whereas, for pharmaceutical substances, it is valid for a period
of 25 years (Moore, 2017). If after getting the patent rights, any other company makes use, sell,
make, import or holding the product for performing any of such actions then it will be considered
as an infringement of the patent rights which is punishable under the law. Such products or
procedures can be used for the purposes mentioned above such as making, selling, importing,
etc. only after consent of the patent holder has been obtained.
Another act, the provisions of which are to be followed by the company is the Trademark Act,
1995. As per the provisions of this act, protection is provided to the trademark of the company
against its use or use of the similar trademark by which the original product cannot be separated
from the other products. Trademark is the word, shape, color, symbol, mark, etc. which is
capable of distinguishing the products and services of one company from the rest of the
companies in the course of trade (Drahos, 2016). Trademarks can only be obtained by applying
to the authority of intellectual property i.e. IP Australia. However, in such case, certain
conditions are to be satisfied such the trademark must be different from the previously registered
trademark. Registration in the Trademark Act provides a right to the holder that only such holder
make use of the trademark, however, such right holder can also authorise other to make use of
such trademark (Gibson, 2017). It results in the infringement of a trademark, if the trademark
right holder is used by other company or person without prior consent of the holder.
Therapeutic Goods Act, 1989 is also to be followed by the company as provides permission for
the formulation of the therapeutic goods and in order to maintain the quality, safety, efficacy and
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

the availability of products being utilised within the boundaries of Australia. Gene Technology
Act, 2000 is also to be complied with by the company. It provides protection to the health and
safety of the people and to the environment against the risks involved in the development of gene
technology.
7
Act, 2000 is also to be complied with by the company. It provides protection to the health and
safety of the people and to the environment against the risks involved in the development of gene
technology.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Question 3
As per the analyses of the AbbVie Inc., it can be determined that it is the originated as a spin-off
of Abbott Laboratories and traded as the biopharmaceutical company. To operate the laws in the
different nation, it is essential for the AbbVie Inc. to comply with the different agreements,
treaties, and conventions. The agreement formed by the company helps to understand the terms
and conditions of the arrangement and that also resolves the conflict among the parties. It also
enhances the smooth running and functioning of the business in different nations. It also
evaluates the conventions that can be organized at the worldwide level by transitions that can
arrange properly among the different conventions.
With the help of agreement, they can acquire, merge, and collaborate the various companies to
perform their functions. AbbVie makes the agreement to acquire the firm of oncology that helps
in the treatment of blood cancers, AstraZeneca, Ibrutinib. AbbVie formed the agreement on
certain terms such as the cost of share, value, completion etc. The name of Pharmacyclics
retained and operates as the subsidiary of AbbVie from the previous industry. They also entered
into the collaboration and licensing agreement that is the written agreement that provides the
rights to the owner to use the property. It also helps to develop and commercialize the products
that help to combine the treatments and Halozyme’s drug delivery technology.
In relation to the Biopharmaceutical sector, there is a convention on Biological Diversity that can
be recognized as the Biodiversity convention and considered as the multilateral treaty. The main
goals of the convention are to conserve the biological diversity and also use the product in a
sustainable manner. They also use the products in a fair and equitable manner. It is signed on 5
June 1992 and entered in to force on 29 December 1993 with 168 nations. This convention is
recognized for the common concern of humankind and also form the part of the development
process. It also helps in the process of decision making in case of threat or loss to the biological
diversity. It consists of various resources such as genetic, ecosystems, species etc. It also
includes the field of biotechnology that is Cartagena protocol that addresses the safety issues. It
provides the advantage to the organization in relation to the services and products that can be
provided to the global market. The different issue is:
8
As per the analyses of the AbbVie Inc., it can be determined that it is the originated as a spin-off
of Abbott Laboratories and traded as the biopharmaceutical company. To operate the laws in the
different nation, it is essential for the AbbVie Inc. to comply with the different agreements,
treaties, and conventions. The agreement formed by the company helps to understand the terms
and conditions of the arrangement and that also resolves the conflict among the parties. It also
enhances the smooth running and functioning of the business in different nations. It also
evaluates the conventions that can be organized at the worldwide level by transitions that can
arrange properly among the different conventions.
With the help of agreement, they can acquire, merge, and collaborate the various companies to
perform their functions. AbbVie makes the agreement to acquire the firm of oncology that helps
in the treatment of blood cancers, AstraZeneca, Ibrutinib. AbbVie formed the agreement on
certain terms such as the cost of share, value, completion etc. The name of Pharmacyclics
retained and operates as the subsidiary of AbbVie from the previous industry. They also entered
into the collaboration and licensing agreement that is the written agreement that provides the
rights to the owner to use the property. It also helps to develop and commercialize the products
that help to combine the treatments and Halozyme’s drug delivery technology.
In relation to the Biopharmaceutical sector, there is a convention on Biological Diversity that can
be recognized as the Biodiversity convention and considered as the multilateral treaty. The main
goals of the convention are to conserve the biological diversity and also use the product in a
sustainable manner. They also use the products in a fair and equitable manner. It is signed on 5
June 1992 and entered in to force on 29 December 1993 with 168 nations. This convention is
recognized for the common concern of humankind and also form the part of the development
process. It also helps in the process of decision making in case of threat or loss to the biological
diversity. It consists of various resources such as genetic, ecosystems, species etc. It also
includes the field of biotechnology that is Cartagena protocol that addresses the safety issues. It
provides the advantage to the organization in relation to the services and products that can be
provided to the global market. The different issue is:
8

It controls the access to genetic resources and also provides the knowledge for the
resources that require the permission of parties who are providing the services of
resources.
This convention measures the encouragements for preserving and satisfying the use of
biological diversity.
It also alerts the public and educates the citizens in some fields.
It also accesses the technology or transfers the technology to the government of state
members.
It also evaluates the impact of the conserving and making the optimum utilization of
resources.
Another convention which was organised in relation to the pharmaceutical sector was the
Pharmaceutical Inspection Convention. The main aim of this scheme was to improve
cooperation and coordination between the pharmaceutical companies and the authorities
overseeing the business activities of such companies in term of practices for good manufacturing.
This convention was conducted in October 1970 by a free trade association of Europe. As the
European Law was not consistent with the convention, new members were unable to be
admitted. This gave rise to the Pharmaceutical Inspection Scheme which was formulated on 2nd
November 1995 (PIC/S, 2018). It is an agreement between the authorities responsible for
overseeing the activities of the company’s manufacturing medicinal products or drugs. It was in
the form of a legal agreement between the authorities regulating the practices for good
manufacturing (GMP). In this arrangement, inspection reports and the certification for GMP is to
be exchanged between the authorities of the members like Australia, United States, etc. This
facilitated the exchange of information between authorities of the members to this convention.
There are various agreements which are signed by the management of the AbbVie Inc. in order
to develop more therapeutic goods for the treatments of different diseases. Such agreements are
made within the national boundaries as well as beyond such boundaries. An agreement is made
between the AbbVie Inc. and Inventiva for the purpose of developing the new potent orally
available small Molecule RORγ Inverse Agonist Drug Candidates (Market Insider, 2017).
Inventiva is a pharmaceutical company having it's headquarters in France and is responsible for
developing or formulating the therapies with the help of innovation, specifically in fibrosis. Such
9
resources that require the permission of parties who are providing the services of
resources.
This convention measures the encouragements for preserving and satisfying the use of
biological diversity.
It also alerts the public and educates the citizens in some fields.
It also accesses the technology or transfers the technology to the government of state
members.
It also evaluates the impact of the conserving and making the optimum utilization of
resources.
Another convention which was organised in relation to the pharmaceutical sector was the
Pharmaceutical Inspection Convention. The main aim of this scheme was to improve
cooperation and coordination between the pharmaceutical companies and the authorities
overseeing the business activities of such companies in term of practices for good manufacturing.
This convention was conducted in October 1970 by a free trade association of Europe. As the
European Law was not consistent with the convention, new members were unable to be
admitted. This gave rise to the Pharmaceutical Inspection Scheme which was formulated on 2nd
November 1995 (PIC/S, 2018). It is an agreement between the authorities responsible for
overseeing the activities of the company’s manufacturing medicinal products or drugs. It was in
the form of a legal agreement between the authorities regulating the practices for good
manufacturing (GMP). In this arrangement, inspection reports and the certification for GMP is to
be exchanged between the authorities of the members like Australia, United States, etc. This
facilitated the exchange of information between authorities of the members to this convention.
There are various agreements which are signed by the management of the AbbVie Inc. in order
to develop more therapeutic goods for the treatments of different diseases. Such agreements are
made within the national boundaries as well as beyond such boundaries. An agreement is made
between the AbbVie Inc. and Inventiva for the purpose of developing the new potent orally
available small Molecule RORγ Inverse Agonist Drug Candidates (Market Insider, 2017).
Inventiva is a pharmaceutical company having it's headquarters in France and is responsible for
developing or formulating the therapies with the help of innovation, specifically in fibrosis. Such
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

an agreement was made to develop and discover for orally available RORγ Inverse Agonist (Le
Prestre, 2017). As the work for developing and making a discovery was already going on, it was
also announced that AbbVie’s current ROR-γ inverse agonist lead compound, ABBV553, will
come to an end in terms of development and its will be followed by Phase 1 study.
Such conventions and agreements are beneficial for the companies operating within the different
legal system and its practices as it provides certain rules or norms which are to be followed
worldwide or within the trade within the member nations. This helps exchange of information,
knowledge, technology, etc. by which the growth of the organisation is confirmed. These
agreements and conventions are beneficial for the growth of AbbVie Inc. and the development of
the researches in which the company is indulged.
10
Prestre, 2017). As the work for developing and making a discovery was already going on, it was
also announced that AbbVie’s current ROR-γ inverse agonist lead compound, ABBV553, will
come to an end in terms of development and its will be followed by Phase 1 study.
Such conventions and agreements are beneficial for the companies operating within the different
legal system and its practices as it provides certain rules or norms which are to be followed
worldwide or within the trade within the member nations. This helps exchange of information,
knowledge, technology, etc. by which the growth of the organisation is confirmed. These
agreements and conventions are beneficial for the growth of AbbVie Inc. and the development of
the researches in which the company is indulged.
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Reference:
AbbVie. (2018). About AbbVie. Available at:
https://www.abbvie.com/our-company/about-abbvie.html. [Accessed on: 17.08.2018]
Drahos, P. (2016). A philosophy of intellectual property. Routledge.
Dutfield, G. (2017). Intellectual property rights and the life science industries: a
twentieth century history. Routledge.
Gibson, J. (2017). Creating selves: intellectual property and the narration of culture.
Routledge.
Le Prestre, P. G. (2017). Governing global biodiversity: The evolution and
implementation of the convention on biological diversity. Routledge.
Market Insider. (2017).
Inventiva and AbbVie Extend Agreement to Discover New Potent Orally-Available
Small Molecule ROR? Inverse Agonist Drug Candidates. Available at:
https://markets.businessinsider.com/news/stocks/inventiva-and-abbvie-extend-agreement-
to-discover-new-potent-orally-available-small-molecule-ror-inverse-agonist-drug-
candidates-1002304746. [Accessed on: 17.08.2018]
Moore, A. (2017). Intellectual property and information control: philosophic foundations
and contemporary issues. Routledge.
PIC/S. (2018). History of PIC/S. Available at: https://picscheme.org/en/history.
[Accessed on: 17.08.2018]
Statista. (2018). AbbVie's number of employees between 2013 and 2018. Available at:
https://www.statista.com/statistics/417023/employees-of-abbvie/. [Accessed on:
17.08.2018]
11
AbbVie. (2018). About AbbVie. Available at:
https://www.abbvie.com/our-company/about-abbvie.html. [Accessed on: 17.08.2018]
Drahos, P. (2016). A philosophy of intellectual property. Routledge.
Dutfield, G. (2017). Intellectual property rights and the life science industries: a
twentieth century history. Routledge.
Gibson, J. (2017). Creating selves: intellectual property and the narration of culture.
Routledge.
Le Prestre, P. G. (2017). Governing global biodiversity: The evolution and
implementation of the convention on biological diversity. Routledge.
Market Insider. (2017).
Inventiva and AbbVie Extend Agreement to Discover New Potent Orally-Available
Small Molecule ROR? Inverse Agonist Drug Candidates. Available at:
https://markets.businessinsider.com/news/stocks/inventiva-and-abbvie-extend-agreement-
to-discover-new-potent-orally-available-small-molecule-ror-inverse-agonist-drug-
candidates-1002304746. [Accessed on: 17.08.2018]
Moore, A. (2017). Intellectual property and information control: philosophic foundations
and contemporary issues. Routledge.
PIC/S. (2018). History of PIC/S. Available at: https://picscheme.org/en/history.
[Accessed on: 17.08.2018]
Statista. (2018). AbbVie's number of employees between 2013 and 2018. Available at:
https://www.statista.com/statistics/417023/employees-of-abbvie/. [Accessed on:
17.08.2018]
11
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





