Lipid Peroxidation Assay: Liver Homogenate and Antioxidant Effects
VerifiedAdded on 2023/04/21
|18
|1940
|285
Practical Assignment
AI Summary
This assignment presents a lipid peroxidation assay conducted to investigate the degradative process in liver tissue and the potential protective effects of antioxidants. The assay measures malondialdehyde (MDA), an indicator of lipid peroxidation, using the thiobarbituric acid reaction. The experiment involved treating liver homogenate with various reagents, including ferrous ions, hydrogen peroxide, catalase, and quercetin (an antioxidant), and measuring the optical density (OD) at 532 nm. The results show variations in lipid peroxidation levels across different test tubes, with the control tube exhibiting the lowest OD and a tube with ferrous ions and hydrogen peroxide showing the highest. The addition of quercetin was used to demonstrate the effects of an antioxidant. Data analysis included the creation of a standard curve for MDA concentration and calculations of mean, standard deviation, and standard error of the mean. The discussion highlights the process of lipid peroxidation, the role of free radicals, the protective effects of antioxidants, and potential experimental limitations.
LIPID PEROPXIDATION ASSAY
[Document subtitle]
FEBRUARY 26, 2019
STUDENT NAME
STUDENT ID
[Document subtitle]
FEBRUARY 26, 2019
STUDENT NAME
STUDENT ID
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

LIPID PEROXIDATION ASSAY 1
Contents
Abstract......................................................................................................................................................................................................................................... 2
Aim................................................................................................................................................................................................................................................ 2
Introduction................................................................................................................................................................................................................................... 2
Materials and Methods................................................................................................................................................................................................................. 3
RESULTS......................................................................................................................................................................................................................................... 4
Discussion.................................................................................................................................................................................................................................... 13
Contents
Abstract......................................................................................................................................................................................................................................... 2
Aim................................................................................................................................................................................................................................................ 2
Introduction................................................................................................................................................................................................................................... 2
Materials and Methods................................................................................................................................................................................................................. 3
RESULTS......................................................................................................................................................................................................................................... 4
Discussion.................................................................................................................................................................................................................................... 13

LIPID PEROXIDATION ASSAY 2
Abstract: The lipid peroxidation is known to be an indicator of a process called oxidative stress within the tissues and cells. The lipid
peroxides generally consisting of aldehydes and the most abundant one are MDA (malondialdehyde). Therefore, the measurement of MDA is
highly accepted as the indicator of the phenomenon of lipid peroxidation. The objective of this experiment is to assay MDA in presence of
hydrochloric acid. Three of the tubes gave very high optical density readings of value 0.142 and it therefore shows the highest level of lipid
peroxidation. The tube 1 is having the minimum level of optical density with signifies the lowest amount of lipoid peroxidation. The same tube
was also used as the control with a small volume of Tris HCL buffer and homogenate of liver.
Aim: The objective of the experiment is to show the process of lipid peroxidation in vital organ such as liver and demonstrate the potential of
some anti oxidant for prevention of such damages.
Introduction: The process of lipid peroxidation happens in several pathophysiological conditions. The process mainly involves stroke,
atherosclerosis, and ageing (Tsikas, 2017, p 17). The process of lipid peroxidation starts when the hydroxyl and superoxide free radicals are
formed. These free radicals are basically some of the unstable molecules of oxygen that has an unpaired electron present in their outer orbital
(Halliwell and Gutteridge, 2015, p na). The production of free radicals happens as the part of normal physiological process and the production is
further gets counter balanced by the presence of anti oxidants that helps in reduction of harmful impact of free radicals (Nimse and Pal, p
27986). There are several diseases that are known to generate oxidative stress in which there is a significant disturbance occurs between the
production of free radicals and antioxidants (Popracet al., 2017, p 294). The fenton reaction is one of the commonly used reaction that generates
Abstract: The lipid peroxidation is known to be an indicator of a process called oxidative stress within the tissues and cells. The lipid
peroxides generally consisting of aldehydes and the most abundant one are MDA (malondialdehyde). Therefore, the measurement of MDA is
highly accepted as the indicator of the phenomenon of lipid peroxidation. The objective of this experiment is to assay MDA in presence of
hydrochloric acid. Three of the tubes gave very high optical density readings of value 0.142 and it therefore shows the highest level of lipid
peroxidation. The tube 1 is having the minimum level of optical density with signifies the lowest amount of lipoid peroxidation. The same tube
was also used as the control with a small volume of Tris HCL buffer and homogenate of liver.
Aim: The objective of the experiment is to show the process of lipid peroxidation in vital organ such as liver and demonstrate the potential of
some anti oxidant for prevention of such damages.
Introduction: The process of lipid peroxidation happens in several pathophysiological conditions. The process mainly involves stroke,
atherosclerosis, and ageing (Tsikas, 2017, p 17). The process of lipid peroxidation starts when the hydroxyl and superoxide free radicals are
formed. These free radicals are basically some of the unstable molecules of oxygen that has an unpaired electron present in their outer orbital
(Halliwell and Gutteridge, 2015, p na). The production of free radicals happens as the part of normal physiological process and the production is
further gets counter balanced by the presence of anti oxidants that helps in reduction of harmful impact of free radicals (Nimse and Pal, p
27986). There are several diseases that are known to generate oxidative stress in which there is a significant disturbance occurs between the
production of free radicals and antioxidants (Popracet al., 2017, p 294). The fenton reaction is one of the commonly used reaction that generates
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

LIPID PEROXIDATION ASSAY 3
hydroxyl radical and therefore will be used in the experiment to start the lipid peroxidation in liver(Liochev, 2018, p 2). The breakdown of the
lipid into malonaldehyde that reacts with the thiobarbuturic acid and forms a pink colored compound will be measured spectroscopically. This
assay is also called as thiobituric acid reaction and the formation of product are known as TBARS (Ghani et al., 2017, p 196).
Materials and Methods: The description of the material and methods was given in the booklet. There were a series of test tubes kept
under incubation at 30 C for half an hour. Addition of appropriate concentration of the reagents was performed in the test tubes. The addition of
liver homogenate was performed very carefully. It is because as soon as the homogenate will be added the reaction will start. The tubes were
then removed from the water and additional amount of care was taken for the removal of clear fluid with the help of pipette.
hydroxyl radical and therefore will be used in the experiment to start the lipid peroxidation in liver(Liochev, 2018, p 2). The breakdown of the
lipid into malonaldehyde that reacts with the thiobarbuturic acid and forms a pink colored compound will be measured spectroscopically. This
assay is also called as thiobituric acid reaction and the formation of product are known as TBARS (Ghani et al., 2017, p 196).
Materials and Methods: The description of the material and methods was given in the booklet. There were a series of test tubes kept
under incubation at 30 C for half an hour. Addition of appropriate concentration of the reagents was performed in the test tubes. The addition of
liver homogenate was performed very carefully. It is because as soon as the homogenate will be added the reaction will start. The tubes were
then removed from the water and additional amount of care was taken for the removal of clear fluid with the help of pipette.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
LIPID PEROXIDATION ASSAY 4
RESULTS
RESULT 1
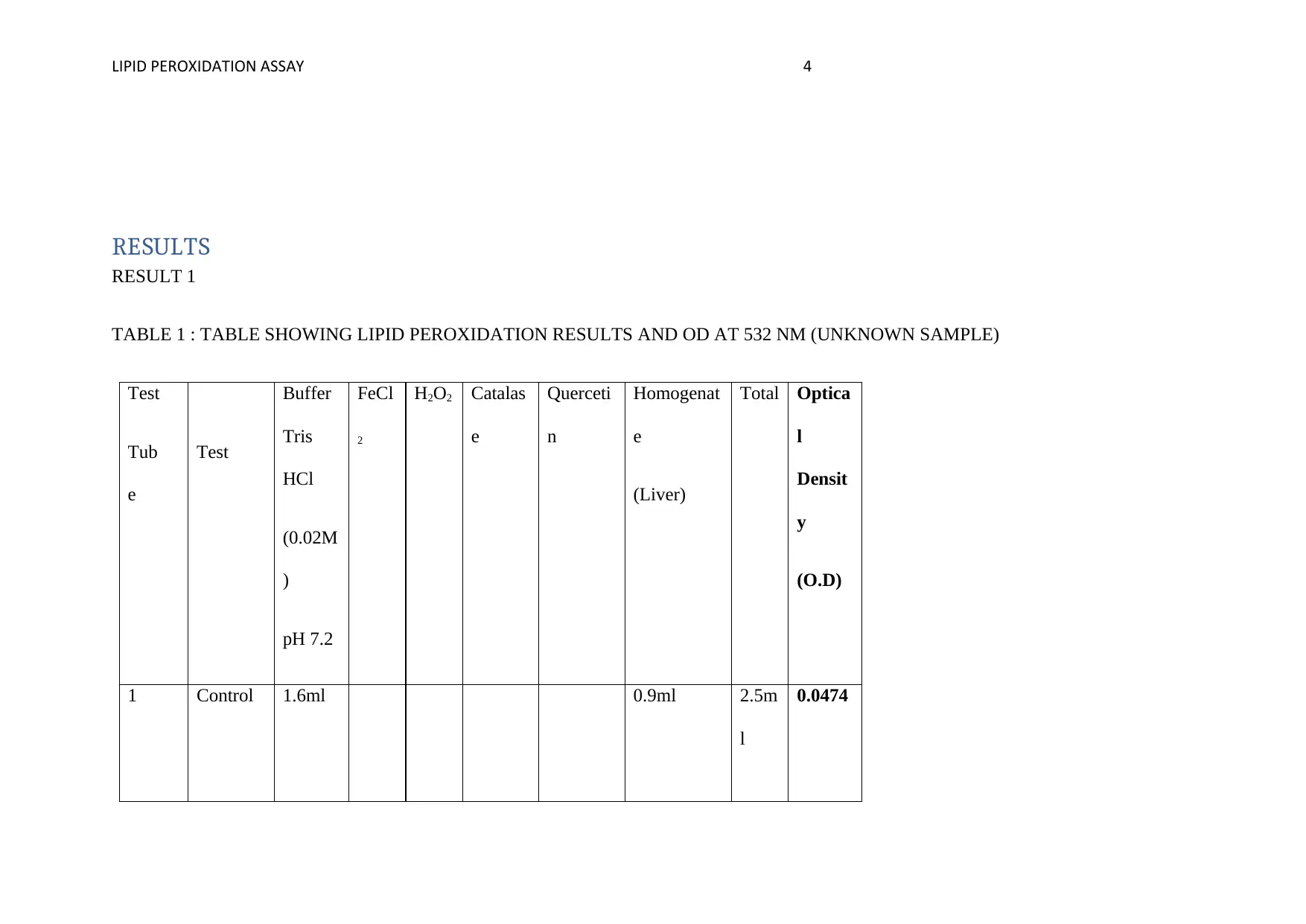
TABLE 1 : TABLE SHOWING LIPID PEROXIDATION RESULTS AND OD AT 532 NM (UNKNOWN SAMPLE)
Test
Tub
e
Test
Buffer
Tris
HCl
(0.02M
)
pH 7.2
FeCl
2
H2O2 Catalas
e
Querceti
n
Homogenat
e
(Liver)
Total Optica
l
Densit
y
(O.D)
1 Control 1.6ml 0.9ml 2.5m
l
0.0474
RESULTS
RESULT 1
TABLE 1 : TABLE SHOWING LIPID PEROXIDATION RESULTS AND OD AT 532 NM (UNKNOWN SAMPLE)
Test
Tub
e
Test
Buffer
Tris
HCl
(0.02M
)
pH 7.2
FeCl
2
H2O2 Catalas
e
Querceti
n
Homogenat
e
(Liver)
Total Optica
l
Densit
y
(O.D)
1 Control 1.6ml 0.9ml 2.5m
l
0.0474
LIPID PEROXIDATION ASSAY 5
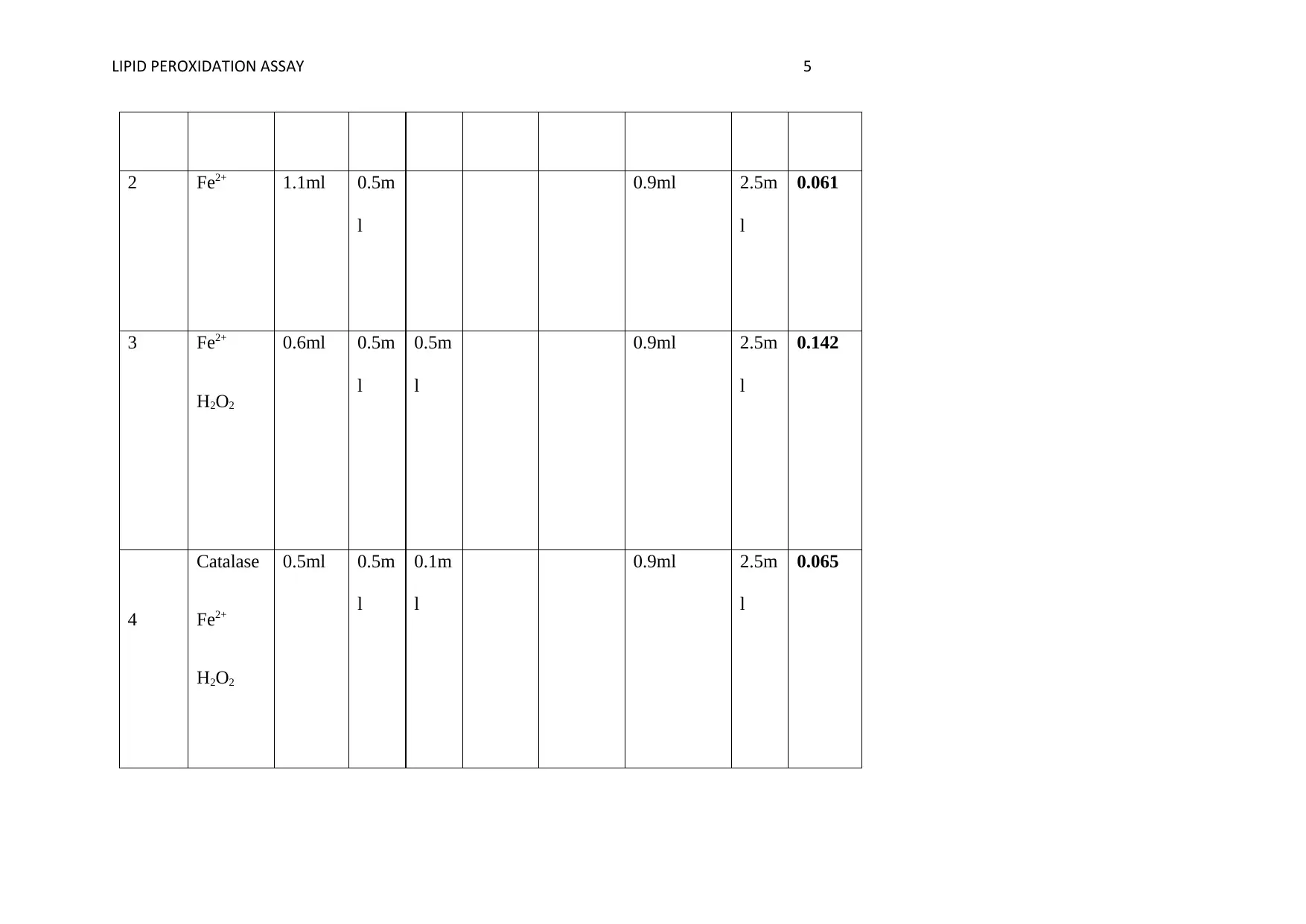
2 Fe2+ 1.1ml 0.5m
l
0.9ml 2.5m
l
0.061
3 Fe2+
H2O2
0.6ml 0.5m
l
0.5m
l
0.9ml 2.5m
l
0.142
4
Catalase
Fe2+
H2O2
0.5ml 0.5m
l
0.1m
l
0.9ml 2.5m
l
0.065
2 Fe2+ 1.1ml 0.5m
l
0.9ml 2.5m
l
0.061
3 Fe2+
H2O2
0.6ml 0.5m
l
0.5m
l
0.9ml 2.5m
l
0.142
4
Catalase
Fe2+
H2O2
0.5ml 0.5m
l
0.1m
l
0.9ml 2.5m
l
0.065
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
LIPID PEROXIDATION ASSAY 6
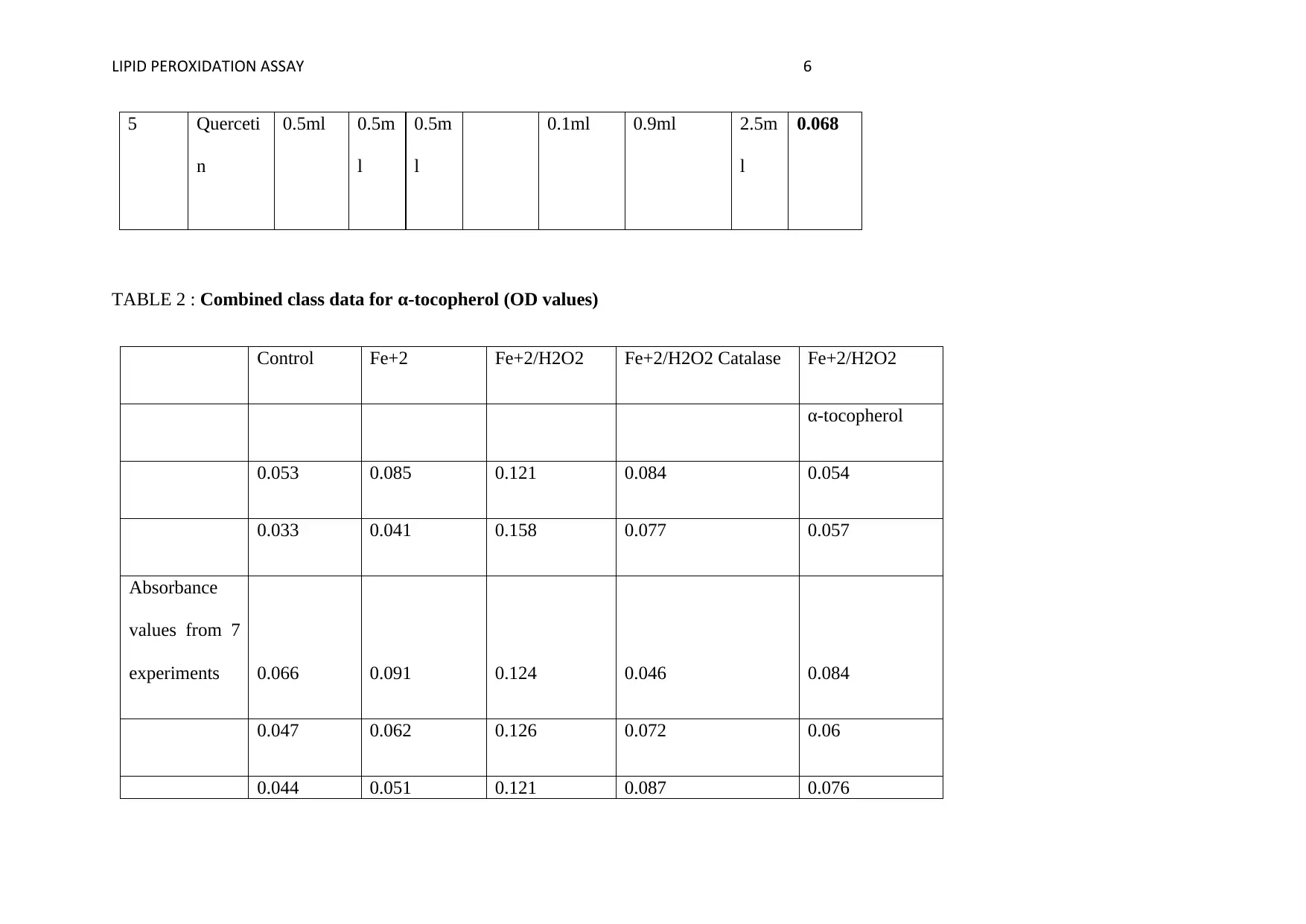
5 Querceti
n
0.5ml 0.5m
l
0.5m
l
0.1ml 0.9ml 2.5m
l
0.068
TABLE 2 : Combined class data for α-tocopherol (OD values)
Control Fe+2 Fe+2/H2O2 Fe+2/H2O2 Catalase Fe+2/H2O2
α-tocopherol
0.053 0.085 0.121 0.084 0.054
0.033 0.041 0.158 0.077 0.057
Absorbance
values from 7
experiments 0.066 0.091 0.124 0.046 0.084
0.047 0.062 0.126 0.072 0.06
0.044 0.051 0.121 0.087 0.076
5 Querceti
n
0.5ml 0.5m
l
0.5m
l
0.1ml 0.9ml 2.5m
l
0.068
TABLE 2 : Combined class data for α-tocopherol (OD values)
Control Fe+2 Fe+2/H2O2 Fe+2/H2O2 Catalase Fe+2/H2O2
α-tocopherol
0.053 0.085 0.121 0.084 0.054
0.033 0.041 0.158 0.077 0.057
Absorbance
values from 7
experiments 0.066 0.091 0.124 0.046 0.084
0.047 0.062 0.126 0.072 0.06
0.044 0.051 0.121 0.087 0.076
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
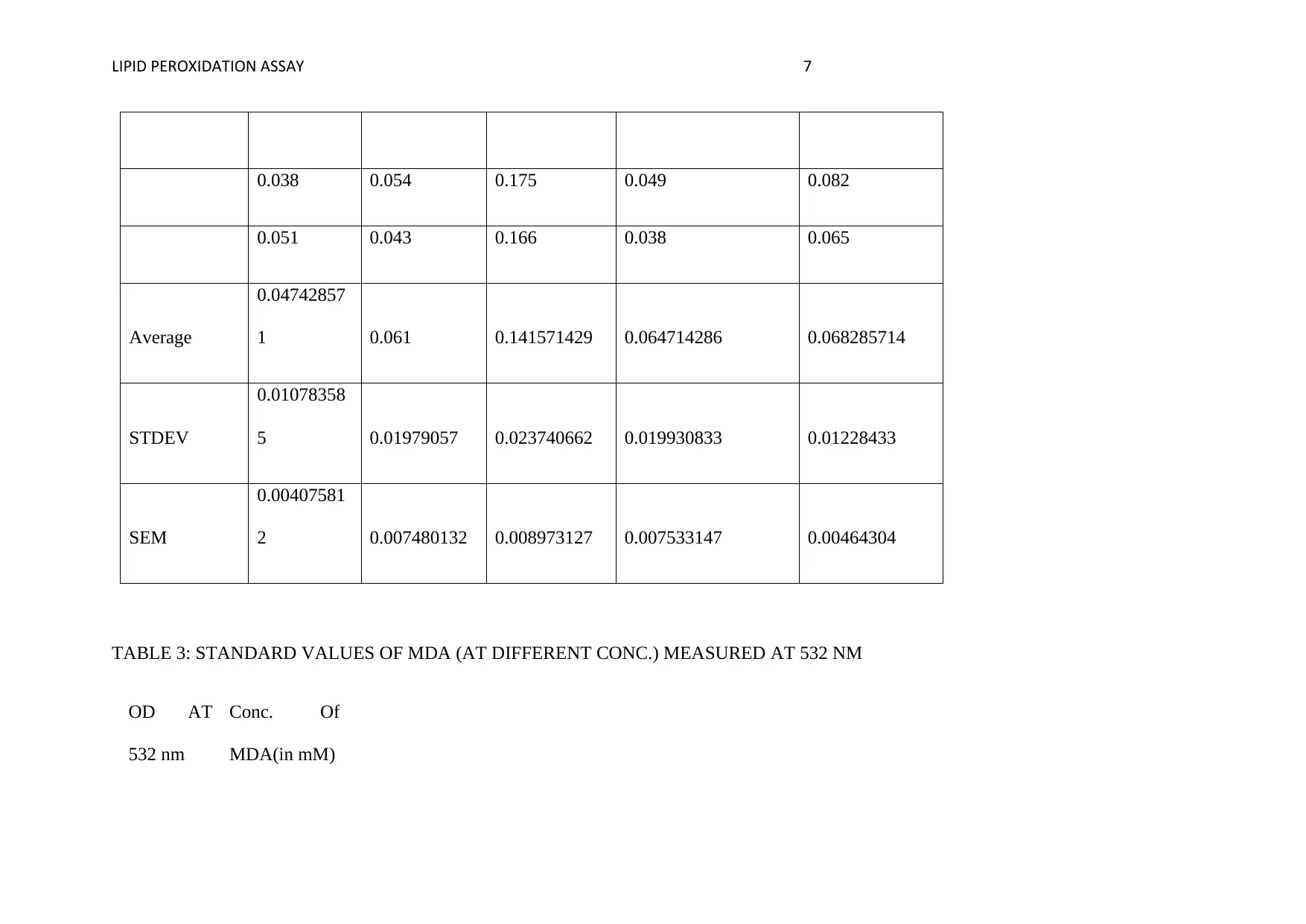
LIPID PEROXIDATION ASSAY 7
0.038 0.054 0.175 0.049 0.082
0.051 0.043 0.166 0.038 0.065
Average
0.04742857
1 0.061 0.141571429 0.064714286 0.068285714
STDEV
0.01078358
5 0.01979057 0.023740662 0.019930833 0.01228433
SEM
0.00407581
2 0.007480132 0.008973127 0.007533147 0.00464304
TABLE 3: STANDARD VALUES OF MDA (AT DIFFERENT CONC.) MEASURED AT 532 NM
OD AT
532 nm
Conc. Of
MDA(in mM)
0.038 0.054 0.175 0.049 0.082
0.051 0.043 0.166 0.038 0.065
Average
0.04742857
1 0.061 0.141571429 0.064714286 0.068285714
STDEV
0.01078358
5 0.01979057 0.023740662 0.019930833 0.01228433
SEM
0.00407581
2 0.007480132 0.008973127 0.007533147 0.00464304
TABLE 3: STANDARD VALUES OF MDA (AT DIFFERENT CONC.) MEASURED AT 532 NM
OD AT
532 nm
Conc. Of
MDA(in mM)

LIPID PEROXIDATION ASSAY 8
0 0
0.065 0.00125
0.13 0.0025
0.26 0.005
0.52 0.01
2.6 0.05
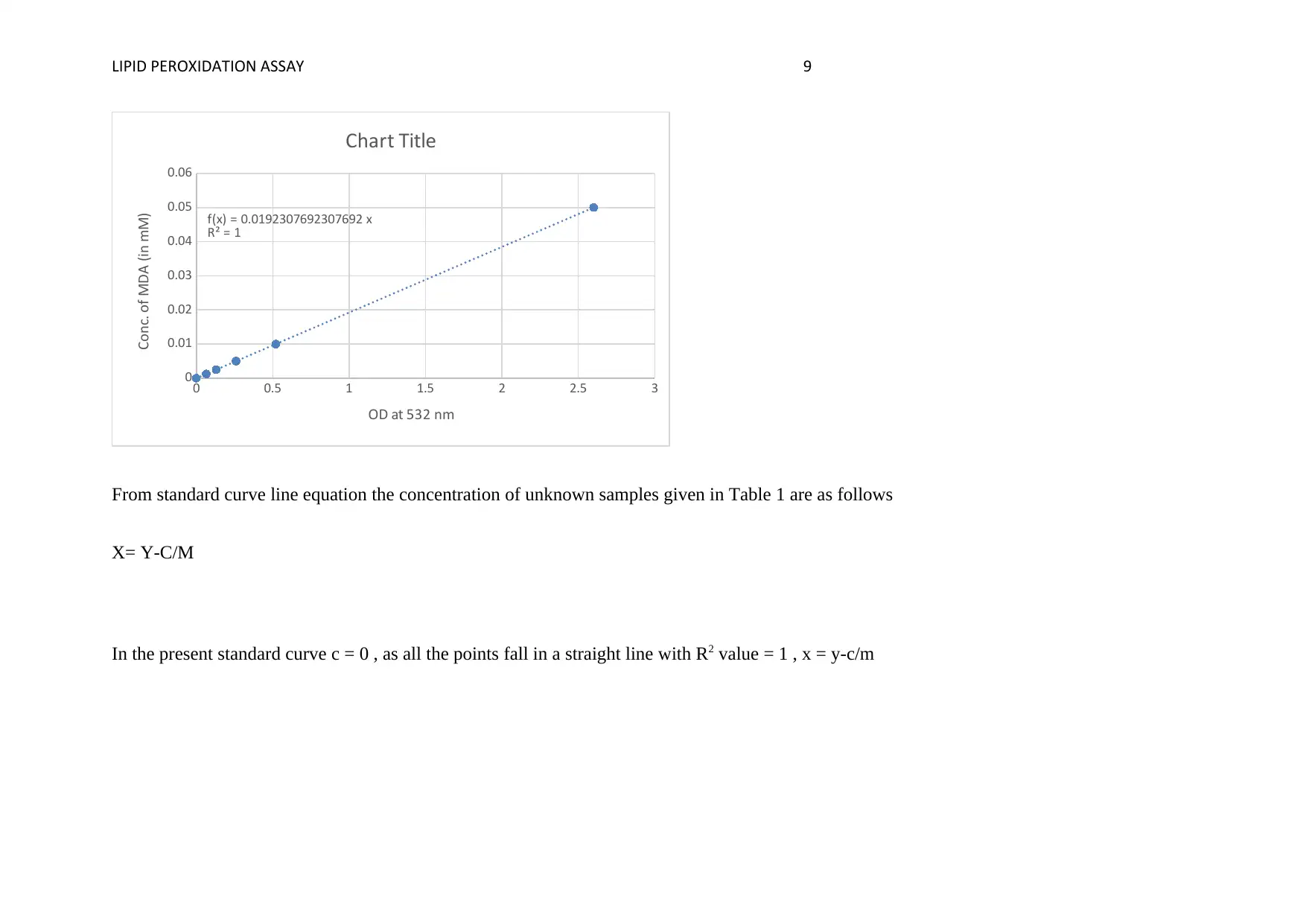
FIG. 1 STANDARD CURVE OF MDA CONCENTRATION BASED ON VALUES OBTAINED IN TABLE 3
0 0
0.065 0.00125
0.13 0.0025
0.26 0.005
0.52 0.01
2.6 0.05
FIG. 1 STANDARD CURVE OF MDA CONCENTRATION BASED ON VALUES OBTAINED IN TABLE 3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
LIPID PEROXIDATION ASSAY 9
0 0.5 1 1.5 2 2.5 3
0
0.01
0.02
0.03
0.04
0.05
0.06
f(x) = 0.0192307692307692 x
R² = 1
Chart Title
OD at 532 nm
Conc. of MDA (in mM)
From standard curve line equation the concentration of unknown samples given in Table 1 are as follows
X= Y-C/M
In the present standard curve c = 0 , as all the points fall in a straight line with R2 value = 1 , x = y-c/m
0 0.5 1 1.5 2 2.5 3
0
0.01
0.02
0.03
0.04
0.05
0.06
f(x) = 0.0192307692307692 x
R² = 1
Chart Title
OD at 532 nm
Conc. of MDA (in mM)
From standard curve line equation the concentration of unknown samples given in Table 1 are as follows
X= Y-C/M
In the present standard curve c = 0 , as all the points fall in a straight line with R2 value = 1 , x = y-c/m
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
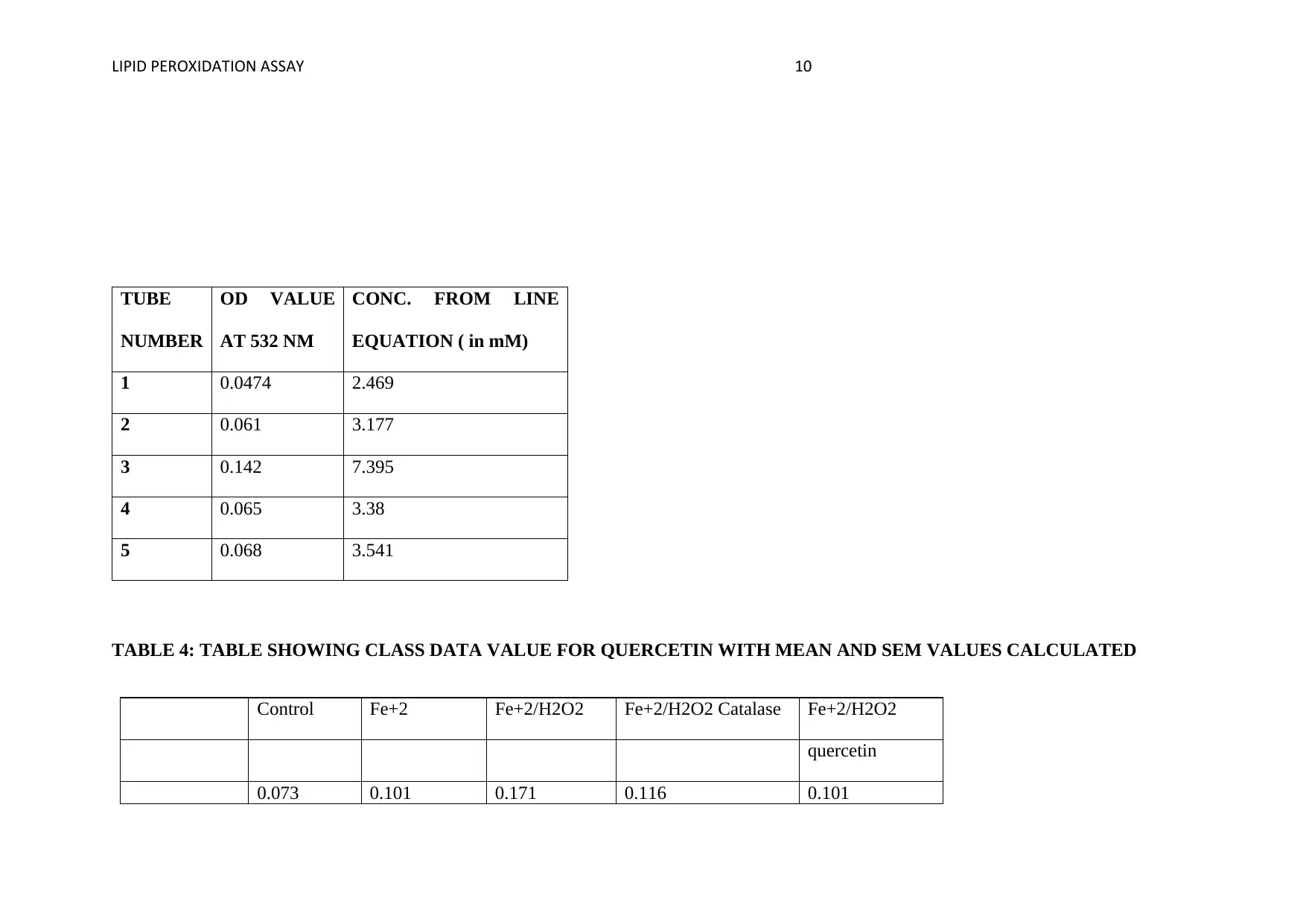
LIPID PEROXIDATION ASSAY 10
TUBE
NUMBER
OD VALUE
AT 532 NM
CONC. FROM LINE
EQUATION ( in mM)
1 0.0474 2.469
2 0.061 3.177
3 0.142 7.395
4 0.065 3.38
5 0.068 3.541
TABLE 4: TABLE SHOWING CLASS DATA VALUE FOR QUERCETIN WITH MEAN AND SEM VALUES CALCULATED
Control Fe+2 Fe+2/H2O2 Fe+2/H2O2 Catalase Fe+2/H2O2
quercetin
0.073 0.101 0.171 0.116 0.101
TUBE
NUMBER
OD VALUE
AT 532 NM
CONC. FROM LINE
EQUATION ( in mM)
1 0.0474 2.469
2 0.061 3.177
3 0.142 7.395
4 0.065 3.38
5 0.068 3.541
TABLE 4: TABLE SHOWING CLASS DATA VALUE FOR QUERCETIN WITH MEAN AND SEM VALUES CALCULATED
Control Fe+2 Fe+2/H2O2 Fe+2/H2O2 Catalase Fe+2/H2O2
quercetin
0.073 0.101 0.171 0.116 0.101
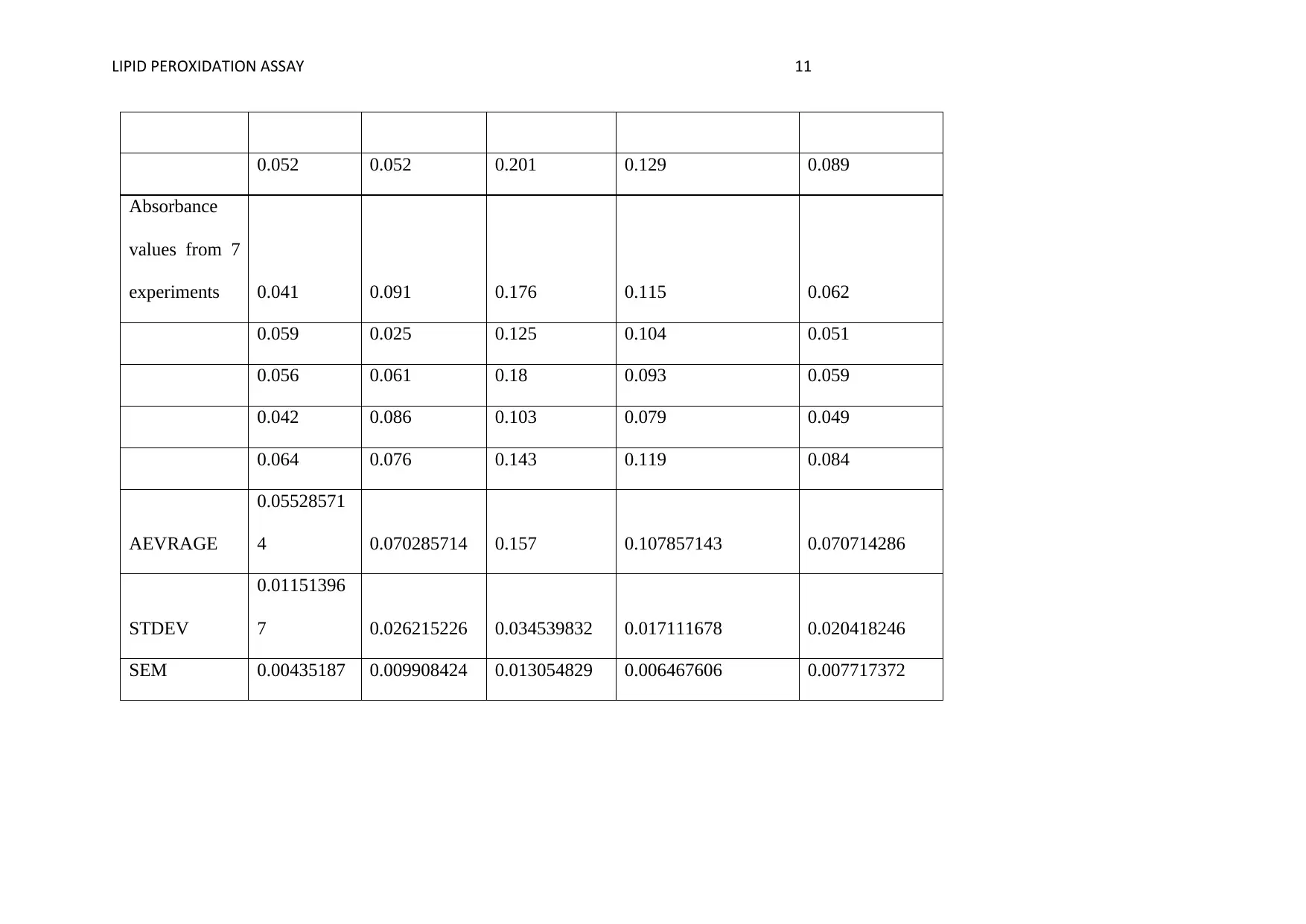
LIPID PEROXIDATION ASSAY 11
0.052 0.052 0.201 0.129 0.089
Absorbance
values from 7
experiments 0.041 0.091 0.176 0.115 0.062
0.059 0.025 0.125 0.104 0.051
0.056 0.061 0.18 0.093 0.059
0.042 0.086 0.103 0.079 0.049
0.064 0.076 0.143 0.119 0.084
AEVRAGE
0.05528571
4 0.070285714 0.157 0.107857143 0.070714286
STDEV
0.01151396
7 0.026215226 0.034539832 0.017111678 0.020418246
SEM 0.00435187 0.009908424 0.013054829 0.006467606 0.007717372
0.052 0.052 0.201 0.129 0.089
Absorbance
values from 7
experiments 0.041 0.091 0.176 0.115 0.062
0.059 0.025 0.125 0.104 0.051
0.056 0.061 0.18 0.093 0.059
0.042 0.086 0.103 0.079 0.049
0.064 0.076 0.143 0.119 0.084
AEVRAGE
0.05528571
4 0.070285714 0.157 0.107857143 0.070714286
STDEV
0.01151396
7 0.026215226 0.034539832 0.017111678 0.020418246
SEM 0.00435187 0.009908424 0.013054829 0.006467606 0.007717372
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 18
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
