Literature Review of Informed Consent in Prehospital Research
VerifiedAdded on 2023/05/29
|12
|4076
|358
Report
AI Summary
This report presents a literature review on informed consent within the context of prehospital research. The study explores the challenges of obtaining informed consent in emergency situations, considering ethical implications and patient autonomy. The review examines various databases, including PubMed, Medline, and Google Scholar, to analyze relevant articles published between 2009 and 2018. The findings highlight the difficulties in balancing patient rights with the need for timely medical intervention, especially in life-threatening situations. The report discusses different consent models, such as deferred consent and proxy consent, and emphasizes the importance of clear communication and patient understanding. The review also underscores the need for local ethics committee consideration and alternative approaches to informed consent in prehospital research. The study concludes with recommendations for future research, emphasizing the need for careful consideration of consent strategies and the potential benefits of alternative approaches to facilitate prehospital research in critical situations. The report emphasizes the need for a balanced approach that respects patient rights while advancing medical knowledge.

Review of Literature on Informed consent in prehospital research.
Student’s Name:
University:
1
Student’s Name:
University:
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
Abstract 3
Introduction: 4
Methods: 4
Review: 6
Recommendations: 9
Conclusion: 10
References: 11
2
Abstract 3
Introduction: 4
Methods: 4
Review: 6
Recommendations: 9
Conclusion: 10
References: 11
2

Abstract:
Aims: The aim of the following study is to conduct a literature review of the articles published
on the topic: Informed consent in prehospital research.
Methods: The search was conducted using various databases such as PubMed, Medline,
CINAHL, EMBASE and Google Scholar. Studies selected were included on the basis of their
fulfilment of the relevant requirements of the research question published in English language.
The studies included the context of informed consent in prehospital research. The articles which
are not a review of the literature are excluded. However, meta-analyses and studies based on
control trials are included in the review.
Results: Ten articles coordinated the qualification criteria. Five concentrated on prehospital
research, two concentrated on the practicality of utilizing an informed consent, and three
inspected states of mind toward the emergency research. Of the examinations concentrating on
prehospital research, three characterized the prehospital as patients of the past or present and
medicinal services suppliers in the hospital; the other three characterized the research as the all-
inclusive community. In spite of the fact that the study is heterogeneous in terms of structures,
settings, and result measures. A few investigations uncovered that persistent gathering and time
to mediation are obstructed when imminent informed consent is required. At last, conceded
consent, despite the fact that embraced keeps on bringing up critical moral issues, especially
identified with the need and timing of divulgence.
Conclusion: This literature review featured the way that each proposition is remarkable and the
strategy for acquiring consent requires cautious thought by nearby morals advisory groups.
Specific consideration must be paid to the utilization of the population chosen for informed
consent. A few examinations featured the need to think about the utilization of options in
contrast to informed consent to empower the lead of prehospital research in life‐threatening
circumstances. Future research ought to assess conclusions on this point.
Keywords: informed consent, prehospital research, emergency research
3
Aims: The aim of the following study is to conduct a literature review of the articles published
on the topic: Informed consent in prehospital research.
Methods: The search was conducted using various databases such as PubMed, Medline,
CINAHL, EMBASE and Google Scholar. Studies selected were included on the basis of their
fulfilment of the relevant requirements of the research question published in English language.
The studies included the context of informed consent in prehospital research. The articles which
are not a review of the literature are excluded. However, meta-analyses and studies based on
control trials are included in the review.
Results: Ten articles coordinated the qualification criteria. Five concentrated on prehospital
research, two concentrated on the practicality of utilizing an informed consent, and three
inspected states of mind toward the emergency research. Of the examinations concentrating on
prehospital research, three characterized the prehospital as patients of the past or present and
medicinal services suppliers in the hospital; the other three characterized the research as the all-
inclusive community. In spite of the fact that the study is heterogeneous in terms of structures,
settings, and result measures. A few investigations uncovered that persistent gathering and time
to mediation are obstructed when imminent informed consent is required. At last, conceded
consent, despite the fact that embraced keeps on bringing up critical moral issues, especially
identified with the need and timing of divulgence.
Conclusion: This literature review featured the way that each proposition is remarkable and the
strategy for acquiring consent requires cautious thought by nearby morals advisory groups.
Specific consideration must be paid to the utilization of the population chosen for informed
consent. A few examinations featured the need to think about the utilization of options in
contrast to informed consent to empower the lead of prehospital research in life‐threatening
circumstances. Future research ought to assess conclusions on this point.
Keywords: informed consent, prehospital research, emergency research
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Introduction:
A wellbeing crisis is where a patient requires medicinal services in a time period limited by
approaching ill wellbeing or death. This regularly starts in the 'prehospital' environment, for
example, in the patient's own place, or in an open area. Wellbeing crises in the pre-healing centre
condition now and then include Ambulance Paramedics, who give human services with
constrained assets. Prehospital research comes in numerous structures and settings. Zones of
research identifying with rescue vehicle paramedics include trailing human services intercessions
and medications, clinical preliminaries, social conduct explore and the epidemiology of
wellbeing records. As indicated in a study that registers furthermore, files investigate
conventions and meta-analyses, the volume of prehospital research being led and has extended in
the course of recent years (Eltorki, Uleryk and Freedman, 2013). Indeed, even basic
investigations utilizing de-distinguished therapeutic records or perception of patient practices
with no analyst cooperation can in any case undermine the protection of the patient in a few
cases (Moore, Moore and Moore, 2009), and raise doubt about the obligation of human services
providers to advise the patient about the factors that will be against any factors that put the
patient self-rule into difficult situation and their privacy.
Objectives:
This study will investigate the informed consent in prehospital research. The procedure of
informed consent is identified which includes the patient's role, his carer, and other required
consent. This review of literature expects to look at the issues encompassing informed consent in
the prehospital research field from a patient, analyst and social insurance supplier point of view
that provide some knowledge into the conduct of prehospital research.
Methods:
Information sources and search strategy
A broad literature search was manually done with the help of databases i.e., PubMed, Medline,
CINAHL, EMBASE, and Google Scholar. The study is based on articles that were published in
different journals between 2009 to 2018, with the exception of articles published before 2009.
Various keywords are used while searching about articles such as informed consent, prehospital
research, emergency research, etc. In order to identify the relevant articles, a manual search was
also performed of relevant bibliographies on articles that are related to the literature review. The
scope of the study is included in the review articles and is kept limited to the pieces of evidence
concerning with the informed consent in prehospital research (refer Table 1).
4
A wellbeing crisis is where a patient requires medicinal services in a time period limited by
approaching ill wellbeing or death. This regularly starts in the 'prehospital' environment, for
example, in the patient's own place, or in an open area. Wellbeing crises in the pre-healing centre
condition now and then include Ambulance Paramedics, who give human services with
constrained assets. Prehospital research comes in numerous structures and settings. Zones of
research identifying with rescue vehicle paramedics include trailing human services intercessions
and medications, clinical preliminaries, social conduct explore and the epidemiology of
wellbeing records. As indicated in a study that registers furthermore, files investigate
conventions and meta-analyses, the volume of prehospital research being led and has extended in
the course of recent years (Eltorki, Uleryk and Freedman, 2013). Indeed, even basic
investigations utilizing de-distinguished therapeutic records or perception of patient practices
with no analyst cooperation can in any case undermine the protection of the patient in a few
cases (Moore, Moore and Moore, 2009), and raise doubt about the obligation of human services
providers to advise the patient about the factors that will be against any factors that put the
patient self-rule into difficult situation and their privacy.
Objectives:
This study will investigate the informed consent in prehospital research. The procedure of
informed consent is identified which includes the patient's role, his carer, and other required
consent. This review of literature expects to look at the issues encompassing informed consent in
the prehospital research field from a patient, analyst and social insurance supplier point of view
that provide some knowledge into the conduct of prehospital research.
Methods:
Information sources and search strategy
A broad literature search was manually done with the help of databases i.e., PubMed, Medline,
CINAHL, EMBASE, and Google Scholar. The study is based on articles that were published in
different journals between 2009 to 2018, with the exception of articles published before 2009.
Various keywords are used while searching about articles such as informed consent, prehospital
research, emergency research, etc. In order to identify the relevant articles, a manual search was
also performed of relevant bibliographies on articles that are related to the literature review. The
scope of the study is included in the review articles and is kept limited to the pieces of evidence
concerning with the informed consent in prehospital research (refer Table 1).
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Inclusion and Exclusion Criteria
The various study articles that were considered in inclusion criteria are the studies that are
literature reviews, meta-analyses or control trials. The studies that are not relevant for the subject
are excluded as well as the studies published before 2009.
Study selection and data extraction
The abstract of the selected articles was studied in order to review whether the studies are
relevant or not. PRISMA flow diagram is used to select the relevant articles (refer Appendix A).
Relevant studies were selected on the basis of the presence of informed setting and their role in
prehospital research and the related data was extracted from each study.
Table 1: Search terms
Pre-hospital informed consent
emergency research
Ethical issues
Clinical trials
Consent
Prehospital
In hospital setting
Results:
Study selection
Ten articles coordinated the qualification criteria. Five concentrated on prehospital research, two
concentrated on the practicality of utilizing an informed consent, and three inspected states of
mind toward the emergency research. Of the examinations concentrating on prehospital research,
three characterized the prehospital as past or current patients and medicinal services suppliers
and administrators in the hospital; the other three characterized the research as the all-inclusive
community.
Informed consent:
Morgans (2010) suggested that the morals of the choice to forgo informed consent depend on the
parity of beneficence (looking for the best wellbeing result for patients), while as yet regarding
patient autonomy (spoke to in their entitlement to settle on their own choices regarding their own
treatment). In any case, there is some legitimacy to the contention that in the event that a patient
5
The various study articles that were considered in inclusion criteria are the studies that are
literature reviews, meta-analyses or control trials. The studies that are not relevant for the subject
are excluded as well as the studies published before 2009.
Study selection and data extraction
The abstract of the selected articles was studied in order to review whether the studies are
relevant or not. PRISMA flow diagram is used to select the relevant articles (refer Appendix A).
Relevant studies were selected on the basis of the presence of informed setting and their role in
prehospital research and the related data was extracted from each study.
Table 1: Search terms
Pre-hospital informed consent
emergency research
Ethical issues
Clinical trials
Consent
Prehospital
In hospital setting
Results:
Study selection
Ten articles coordinated the qualification criteria. Five concentrated on prehospital research, two
concentrated on the practicality of utilizing an informed consent, and three inspected states of
mind toward the emergency research. Of the examinations concentrating on prehospital research,
three characterized the prehospital as past or current patients and medicinal services suppliers
and administrators in the hospital; the other three characterized the research as the all-inclusive
community.
Informed consent:
Morgans (2010) suggested that the morals of the choice to forgo informed consent depend on the
parity of beneficence (looking for the best wellbeing result for patients), while as yet regarding
patient autonomy (spoke to in their entitlement to settle on their own choices regarding their own
treatment). In any case, there is some legitimacy to the contention that in the event that a patient
5

cannot agree to cooperate, the exploration ought to be directed with another participant gathering
or in some other setting which permits assent (Saver et al., 2006).
Directing prehospital research whilst as yet regarding patient self-governance as informed
consent is hindered by 2 factors; potential debilitation of the basic leadership limit of the patient
and the requirement to convey the exploration mediation in the most limited time conceivable.
Additionally, they want to forego consent and recognizes that requesting patient-consent in
restorative crises could be believed to be an unjustifiable weight on an as of now vulnerable
patient (Cole, Ho and Biros, 2016). The basic leadership limit of any patients that experiences a
wellbeing crisis can be influenced by numerous elements, which includes drugs, stress, feeling,
and the disease or damage which caused the crisis. An ongoing article which inspected the ethics
of informed consent for restorative treatment that includes six noteworthy hindrances to
informed consent for therapeutic treatment in the prehospital incidents (Isles, 2013) which are
likewise confronted when looking for informed consent for prehospital research.
The main key component of informed consent is an exposure of the data about the preliminary,
includes both the positive and negative results. This is troublesome to carry out in an opportune
way and postpones the execution of the exploration intercession while it is clarified. This is a
concern in prehospital research, as the opportunity for a few mediations is confined by the
advancement of the better condition of the patient. As the outrageous weight of time experienced
in numerous prehospital incidents (Isles, 2013), using informative explanations in crisis
wellbeing research isn't constantly possible. However, precisely the amount of data that should
be provided to the patient in order to understand the dangers and advantages of the of the study
are also suggested (Thompson, 2003).
(Lie and Witteveen, 2015) discussed in their study about the information steps that the patient
should take and the preferences regarding the consent in order to take part in the research were
explored in a Swedish study which consists of thirty one heart patients. The investigation found
that notwithstanding broadly informed consent processes amid the preliminary's enrolment,
around 2 weeks after the preliminary started the participants had held small comprehension of
the preliminary and their cooperation.
Rawbone, (2010) exploration suggests that notwithstanding when informed consent, the
comprehension, and maintenance of data by the patients and legitimate agents, especially while
patients are intensely not well or under medication, was endangered. Regardless of these
boundaries to the total honesty of data, improvement conveyance to a basic, justifiable
organization would increase the arrangement of data to patients as well as permit consent for
incorporation in a prehospital research trial which is overlooked for where inquire about ideas
are generally uncomplicated.
6
or in some other setting which permits assent (Saver et al., 2006).
Directing prehospital research whilst as yet regarding patient self-governance as informed
consent is hindered by 2 factors; potential debilitation of the basic leadership limit of the patient
and the requirement to convey the exploration mediation in the most limited time conceivable.
Additionally, they want to forego consent and recognizes that requesting patient-consent in
restorative crises could be believed to be an unjustifiable weight on an as of now vulnerable
patient (Cole, Ho and Biros, 2016). The basic leadership limit of any patients that experiences a
wellbeing crisis can be influenced by numerous elements, which includes drugs, stress, feeling,
and the disease or damage which caused the crisis. An ongoing article which inspected the ethics
of informed consent for restorative treatment that includes six noteworthy hindrances to
informed consent for therapeutic treatment in the prehospital incidents (Isles, 2013) which are
likewise confronted when looking for informed consent for prehospital research.
The main key component of informed consent is an exposure of the data about the preliminary,
includes both the positive and negative results. This is troublesome to carry out in an opportune
way and postpones the execution of the exploration intercession while it is clarified. This is a
concern in prehospital research, as the opportunity for a few mediations is confined by the
advancement of the better condition of the patient. As the outrageous weight of time experienced
in numerous prehospital incidents (Isles, 2013), using informative explanations in crisis
wellbeing research isn't constantly possible. However, precisely the amount of data that should
be provided to the patient in order to understand the dangers and advantages of the of the study
are also suggested (Thompson, 2003).
(Lie and Witteveen, 2015) discussed in their study about the information steps that the patient
should take and the preferences regarding the consent in order to take part in the research were
explored in a Swedish study which consists of thirty one heart patients. The investigation found
that notwithstanding broadly informed consent processes amid the preliminary's enrolment,
around 2 weeks after the preliminary started the participants had held small comprehension of
the preliminary and their cooperation.
Rawbone, (2010) exploration suggests that notwithstanding when informed consent, the
comprehension, and maintenance of data by the patients and legitimate agents, especially while
patients are intensely not well or under medication, was endangered. Regardless of these
boundaries to the total honesty of data, improvement conveyance to a basic, justifiable
organization would increase the arrangement of data to patients as well as permit consent for
incorporation in a prehospital research trial which is overlooked for where inquire about ideas
are generally uncomplicated.
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Armstrong et al., (2017) discussed the primary qualities of the 56 papers incorporated into their
study, to be specific countries, condition, and intercession, are abridged. According to their study
countries whose native language is English are the most in the papers however this was not given
the dialect impediments of the study. Similarly, a much more multicentre papers (12, 21.4%),
were also attempted in excess of one country. A few investigations included neighbouring
nations while others incorporated an extensive variety of focuses; one examination had 12 look
into focuses countries worldwide (Montalescot et al., 2013).
Another study by Chin et al., (2015) investigated generally the crisis circumstances in a
prehospital research, with cardiovascular conditions (28 considers, 49.4%, for example, heart
failure happening most regularly, trailed by stroke (7 thinks about, 12.5%) and bring down
hazard wounds and diseases. Drug trials (24 ponders, 42.9%) typically included the early
organization of medications regularly given on arrival in clinic, correlations of two routinely
utilized medications or novel operators, for instance, 100 percent oxygen to avert movement
infection amid transport. Furthermore, gadget preliminaries (12 contemplates, 21.4%) included
correlation of various aviation routes gadgets or the utilization of robotized chest pressure units
in contrast with chest compressions manually (Sandman and Nordmark, 2006). Thirdly, elective
pathway preliminaries (10 thinks about, 17.9%) tried to alleviate the weights on crisis offices by
steering patients to network care or specifically to in doctor's facility treatment. At last, elective
process preliminaries (10 contemplates, 17.9%) surveyed the utilization of various systems in the
rescue vehicle setting, for instance the utilization of CPR preceding defibrillation. The majority
of the papers explored examined strategies used to pick up informed consent to some degree. In a
request to create an account examination of the information, correlations were made between the
sort of assent utilized and the nation in which the investigation occurred, the condition under
scrutiny and the intercession utilized. The kind of consent will have been impacted by the
enactment and controls of the nation being referred to, albeit a few papers referred to worldwide
rules, for example, the Declaration of Helsinki or the Good Clinical Practice (GCP) rules
(Ankolekar et al., 2013).
Multicentre study by Steg et al., (2013) suggested that, in general, utilize a solitary type of
consent, with just two of the twelve papers that utilizes informed consent. An examination of the
informed consent along with the condition being contemplated demonstrated that where patients
needed limit with regards to precedent because of heart failure, informed consent was the most
generally utilized model. The patients who endure the underlying ailment, require consent for
follow-up information accumulation was regularly utilized (Benger et al., 2016). In the following
cases, there was likewise normally arrangement for relative intermediary consent or deferred
consent whereby patients were requested to finish a consent frame when they recouped limit..
The study by Kim et al., (2014) provided data regarding the preliminaries including stroke
patients would, in general, have the most fluctuated consent models and typically included in
excess of one sort of consent, mirroring the changing seriousness of strokes, and the intricate
idea of the condition. This was shown as, while there were just 7-stroke preliminaries detailed,
7
study, to be specific countries, condition, and intercession, are abridged. According to their study
countries whose native language is English are the most in the papers however this was not given
the dialect impediments of the study. Similarly, a much more multicentre papers (12, 21.4%),
were also attempted in excess of one country. A few investigations included neighbouring
nations while others incorporated an extensive variety of focuses; one examination had 12 look
into focuses countries worldwide (Montalescot et al., 2013).
Another study by Chin et al., (2015) investigated generally the crisis circumstances in a
prehospital research, with cardiovascular conditions (28 considers, 49.4%, for example, heart
failure happening most regularly, trailed by stroke (7 thinks about, 12.5%) and bring down
hazard wounds and diseases. Drug trials (24 ponders, 42.9%) typically included the early
organization of medications regularly given on arrival in clinic, correlations of two routinely
utilized medications or novel operators, for instance, 100 percent oxygen to avert movement
infection amid transport. Furthermore, gadget preliminaries (12 contemplates, 21.4%) included
correlation of various aviation routes gadgets or the utilization of robotized chest pressure units
in contrast with chest compressions manually (Sandman and Nordmark, 2006). Thirdly, elective
pathway preliminaries (10 thinks about, 17.9%) tried to alleviate the weights on crisis offices by
steering patients to network care or specifically to in doctor's facility treatment. At last, elective
process preliminaries (10 contemplates, 17.9%) surveyed the utilization of various systems in the
rescue vehicle setting, for instance the utilization of CPR preceding defibrillation. The majority
of the papers explored examined strategies used to pick up informed consent to some degree. In a
request to create an account examination of the information, correlations were made between the
sort of assent utilized and the nation in which the investigation occurred, the condition under
scrutiny and the intercession utilized. The kind of consent will have been impacted by the
enactment and controls of the nation being referred to, albeit a few papers referred to worldwide
rules, for example, the Declaration of Helsinki or the Good Clinical Practice (GCP) rules
(Ankolekar et al., 2013).
Multicentre study by Steg et al., (2013) suggested that, in general, utilize a solitary type of
consent, with just two of the twelve papers that utilizes informed consent. An examination of the
informed consent along with the condition being contemplated demonstrated that where patients
needed limit with regards to precedent because of heart failure, informed consent was the most
generally utilized model. The patients who endure the underlying ailment, require consent for
follow-up information accumulation was regularly utilized (Benger et al., 2016). In the following
cases, there was likewise normally arrangement for relative intermediary consent or deferred
consent whereby patients were requested to finish a consent frame when they recouped limit..
The study by Kim et al., (2014) provided data regarding the preliminaries including stroke
patients would, in general, have the most fluctuated consent models and typically included in
excess of one sort of consent, mirroring the changing seriousness of strokes, and the intricate
idea of the condition. This was shown as, while there were just 7-stroke preliminaries detailed,
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

stroke represented eighteen models of consent. The majority of preliminaries where patients
were probably going to have the limit, even in a crisis circumstance, requested that patients give
composed informed consent in the prehospital research setting (normally in the rescue vehicle).
A few investigations featured by Snooks et al., (2010) suggested that notwithstanding when a
patient was cognizant factors, for example, agony could impact their ability to give informed
consent.
Discussion:
This review investigated 10 studies that attempted in the prehospital research (or comparable) for
a scope of conditions and mediations. Informed consent was the principal thought when
revealing moral issues around clinical preliminaries attempted in the prehospital research.
Investigation of the techniques for acquiring consent demonstrated connections between the sort
of consent and both the condition and the intercession being evaluated.
A considerable lot of the studies investigated contained explanations in regards to ethical
approvals, with a few incorporating proclamations in regards to consistence with worldwide
direction, specifically the Declaration of Helsinki or Good Clinical Practice (GCP) rules
(Buckley, Irving and Goodacre, 2016). Prehospital research setting may subsequently require an
alternate way to deal with informed consent, regardless of whether this is through a widening of
exemption from agree to incorporate circumstances not classed as therapeutic crises or through
an alternate methodology completely, for example, consent. In spite of the fact that this review is
particularly focused on consent there are a scope of other ethical concerns. Aspects, for example,
understanding discernments and expert perspectives of prehospital look into have started to be
investigated in past examinations; these and other ethical issues of emergencies. The equalization
of participation and advantages or value of cooperation ought to be tended to in future
investigations with the end goal to manage future research plan (Davies et al., 2014).
Conclusion:
The search procedure was far-reaching, however, it was recognized that since it was restricted to
access databases and papers written in the English dialect every pertinent paper might not have
been distinguished.
This precise review of the literature found that the capacity to get informed consent was the
overall ethical thought. The review prompts questions with respect to the capacity to acquire
composed informed consent. Regardless of whether elective strategies, for example, more
extensive utilization of emergency case from consent, or informed consent combined with
agreeing to incorporate follow-up information gathering ought to be standard for this sort of
research is indistinct. The utilization of wording to depict consent models was likewise
8
were probably going to have the limit, even in a crisis circumstance, requested that patients give
composed informed consent in the prehospital research setting (normally in the rescue vehicle).
A few investigations featured by Snooks et al., (2010) suggested that notwithstanding when a
patient was cognizant factors, for example, agony could impact their ability to give informed
consent.
Discussion:
This review investigated 10 studies that attempted in the prehospital research (or comparable) for
a scope of conditions and mediations. Informed consent was the principal thought when
revealing moral issues around clinical preliminaries attempted in the prehospital research.
Investigation of the techniques for acquiring consent demonstrated connections between the sort
of consent and both the condition and the intercession being evaluated.
A considerable lot of the studies investigated contained explanations in regards to ethical
approvals, with a few incorporating proclamations in regards to consistence with worldwide
direction, specifically the Declaration of Helsinki or Good Clinical Practice (GCP) rules
(Buckley, Irving and Goodacre, 2016). Prehospital research setting may subsequently require an
alternate way to deal with informed consent, regardless of whether this is through a widening of
exemption from agree to incorporate circumstances not classed as therapeutic crises or through
an alternate methodology completely, for example, consent. In spite of the fact that this review is
particularly focused on consent there are a scope of other ethical concerns. Aspects, for example,
understanding discernments and expert perspectives of prehospital look into have started to be
investigated in past examinations; these and other ethical issues of emergencies. The equalization
of participation and advantages or value of cooperation ought to be tended to in future
investigations with the end goal to manage future research plan (Davies et al., 2014).
Conclusion:
The search procedure was far-reaching, however, it was recognized that since it was restricted to
access databases and papers written in the English dialect every pertinent paper might not have
been distinguished.
This precise review of the literature found that the capacity to get informed consent was the
overall ethical thought. The review prompts questions with respect to the capacity to acquire
composed informed consent. Regardless of whether elective strategies, for example, more
extensive utilization of emergency case from consent, or informed consent combined with
agreeing to incorporate follow-up information gathering ought to be standard for this sort of
research is indistinct. The utilization of wording to depict consent models was likewise
8

exceptionally changed and institutionalization of phrasing would be valuable for the lucidity of
informed consent (for the two members and examiners) and moral contemplations in prehospital
clinical research.
9
informed consent (for the two members and examiners) and moral contemplations in prehospital
clinical research.
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References:
Ankolekar, S., Fuller, M., Cross, I., Renton, C., Cox, P., Sprigg, N., Siriwardena, A. and Bath, P.
(2013). Feasibility of an Ambulance-Based Stroke Trial, and Safety of Glyceryl Trinitrate
in Ultra-Acute Stroke. Stroke, 44(11), pp.3120-3128.
Armstrong, S., Langlois, A., Laparidou, D., Dixon, M., Appleton, J., Bath, P., Snooks, H. and
Siriwardena, A. (2017). Assessment of consent models as an ethical consideration in the
conduct of prehospital ambulance randomised controlled clinical trials: a systematic
review. BMC Medical Research Methodology, 17(1).
Benger, J., Coates, D., Davies, S., Greenwood, R., Nolan, J., Rhys, M., Thomas, M. and Voss, S.
(2016). Randomised comparison of the effectiveness of the laryngeal mask airway
supreme, i-gel and current practice in the initial airway management of out of hospital
cardiac arrest: a feasibility study. British Journal of Anaesthesia, 116(2), pp.262-268.
Buckley, J., Irving, A. and Goodacre, S. (2016). How do patients feel about taking part in clinical
trials in emergency care?: Table 1. Emergency Medicine Journal, 33(6), pp.376-380.
Chin, T., Moore, E., Coors, M., Chandler, J., Ghasabyan, A., Harr, J., Stringham, J., Ramos, C.,
Ammons, S., Banerjee, A. and Sauaia, A. (2015). Exploring ethical conflicts in
emergency trauma research: The COMBAT (Control of Major Bleeding after Trauma)
study experience. Surgery, 157(1), pp.10-19.
Cole, J., Ho, J. and Biros, M. (2016). Randomizing Patients without Consent: Waiver vs
Exception from Informed Consent. Prehospital and Disaster Medicine, 31(04), pp.457-458.
Davies, H., Shakur, H., Padkin, A., Roberts, I., Slowther, A. and Perkins, G. (2014). Guide to the
design and review of emergency research when it is proposed that consent and
consultation be waived: Table 1. Emergency Medicine Journal, 31(10), pp.794-795.
Eltorki, M., Uleryk, E. and Freedman, S. (2013). Waiver of Informed Consent in Pediatric
Resuscitation Research: A Systematic Review. Academic Emergency Medicine, 20(8),
pp.822-834.
Isles, A. (2013). Understood Consent Versus Informed Consent: A New Paradigm for Obtaining
Consent for Pediatric Research Studies. Frontiers in Pediatrics, 1.
Kim, F., Nichol, G., Maynard, C., Hallstrom, A., Kudenchuk, P., Rea, T., Copass, M., Carlbom,
D., Deem, S., Longstreth, W., Olsufka, M. and Cobb, L. (2014). Effect of Prehospital Induction
of Mild Hypothermia on Survival and Neurological Status Among Adults With Cardiac Arrest.
JAMA, 311(1), p.45.
Lie, R. and Witteveen, L. (2015). Visual informed consent: informed consent without forms.
International Journal of Social Research Methodology, 20(1), pp.63-75.
10
Ankolekar, S., Fuller, M., Cross, I., Renton, C., Cox, P., Sprigg, N., Siriwardena, A. and Bath, P.
(2013). Feasibility of an Ambulance-Based Stroke Trial, and Safety of Glyceryl Trinitrate
in Ultra-Acute Stroke. Stroke, 44(11), pp.3120-3128.
Armstrong, S., Langlois, A., Laparidou, D., Dixon, M., Appleton, J., Bath, P., Snooks, H. and
Siriwardena, A. (2017). Assessment of consent models as an ethical consideration in the
conduct of prehospital ambulance randomised controlled clinical trials: a systematic
review. BMC Medical Research Methodology, 17(1).
Benger, J., Coates, D., Davies, S., Greenwood, R., Nolan, J., Rhys, M., Thomas, M. and Voss, S.
(2016). Randomised comparison of the effectiveness of the laryngeal mask airway
supreme, i-gel and current practice in the initial airway management of out of hospital
cardiac arrest: a feasibility study. British Journal of Anaesthesia, 116(2), pp.262-268.
Buckley, J., Irving, A. and Goodacre, S. (2016). How do patients feel about taking part in clinical
trials in emergency care?: Table 1. Emergency Medicine Journal, 33(6), pp.376-380.
Chin, T., Moore, E., Coors, M., Chandler, J., Ghasabyan, A., Harr, J., Stringham, J., Ramos, C.,
Ammons, S., Banerjee, A. and Sauaia, A. (2015). Exploring ethical conflicts in
emergency trauma research: The COMBAT (Control of Major Bleeding after Trauma)
study experience. Surgery, 157(1), pp.10-19.
Cole, J., Ho, J. and Biros, M. (2016). Randomizing Patients without Consent: Waiver vs
Exception from Informed Consent. Prehospital and Disaster Medicine, 31(04), pp.457-458.
Davies, H., Shakur, H., Padkin, A., Roberts, I., Slowther, A. and Perkins, G. (2014). Guide to the
design and review of emergency research when it is proposed that consent and
consultation be waived: Table 1. Emergency Medicine Journal, 31(10), pp.794-795.
Eltorki, M., Uleryk, E. and Freedman, S. (2013). Waiver of Informed Consent in Pediatric
Resuscitation Research: A Systematic Review. Academic Emergency Medicine, 20(8),
pp.822-834.
Isles, A. (2013). Understood Consent Versus Informed Consent: A New Paradigm for Obtaining
Consent for Pediatric Research Studies. Frontiers in Pediatrics, 1.
Kim, F., Nichol, G., Maynard, C., Hallstrom, A., Kudenchuk, P., Rea, T., Copass, M., Carlbom,
D., Deem, S., Longstreth, W., Olsufka, M. and Cobb, L. (2014). Effect of Prehospital Induction
of Mild Hypothermia on Survival and Neurological Status Among Adults With Cardiac Arrest.
JAMA, 311(1), p.45.
Lie, R. and Witteveen, L. (2015). Visual informed consent: informed consent without forms.
International Journal of Social Research Methodology, 20(1), pp.63-75.
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Montalescot, G., Lassen, J., Hamm, C., Lapostolle, F., Silvain, J., ten Berg, J., Cantor, W., Goodman, S.,
Licour, M., Tsatsaris, A. and van't Hof, A. (2013). Ambulance or in-catheterization laboratory
administration of ticagrelor for primary percutaneous coronary intervention for ST-segment
elevation myocardial infarction: Rationale and design of the randomized, double-blind
Administration of Ticagrelor in the cath Lab or in the Ambulance for New ST elevation
myocardial Infarction to open the Coronary artery (ATLANTIC) study. American Heart Journal,
165(4), pp.515-522.
Moore, P., Moore, E. and Moore, F. (2009). Exception from informed consent requirements for
emergency research. Surgery, 145(6), pp.630-635.
Morgans, A. (2010). Waiver of Informed Consent in Prehospital Emergency Health Research in
Australia. Monash Bioethics Review, 29(1), pp.49-64.
Rawbone, R. (2010). Consent: Informed or Not Informed, That is the Question. Research Ethics, 6(4),
pp.115-116.
Sandman, L. and Nordmark, A. (2006). Ethical Conflicts in Prehospital Emergency Care. Nursing
Ethics, 13(6), pp.592-607.
Saver, J., Kidwell, C., Eckstein, M., Ovbiagele, B. and Starkman, S. (2006). Physician-Investigator
Phone Elicitation of Consent in the Field: A Novel Method To Obtain Explicit Informed Consent
For Prehospital Clinical Research. Prehospital Emergency Care, 10(2), pp.182-185.
Snooks, H., Cheung, W., Close, J., Dale, J., Gaze, S., Humphreys, I., Lyons, R., Mason, S., Merali, Y.,
Peconi, J., Phillips, C., Phillips, J., Roberts, S., Russell, I., Sánchez, A., Wani, M., Wells, B. and
Whitfield, R. (2010). Support and Assessment for Fall Emergency Referrals (SAFER 1) trial
protocol. Computerised on-scene decision support for emergency ambulance staff to assess and
plan care for older people who have fallen: evaluation of costs and benefits using a pragmatic
cluster randomised trial. BMC Emergency Medicine, 10(1).
Steg, P., van ‘t Hof, A., Clemmensen, P., Lapostolle, F., Dudek, D., Hamon, M., Cavallini, C., Gordini,
G., Huber, K., Coste, P., Thicoipe, M., Nibbe, L., Steinmetz, J., Ten Berg, J., Eggink, G.,
Zeymer, U., Campo dell' Orto, M., Kanic, V., Deliargyris, E., Day, J., Schuette, D., Hamm, C.
and Goldstein, P. (2013). Design and methods of European Ambulance Acute Coronary
Syndrome Angiography Trial (EUROMAX): An international randomized open-label ambulance
trial of bivalirudin versus standard-of-care anticoagulation in patients with acute ST-segment-
elevation myocardial infarction transferred for primary percutaneous coronary intervention.
American Heart Journal, 166(6), pp.960-967.e6.
Thompson, J. (2003). Ethical challenges of informed consent in prehospital research. CJEM, 5(02),
pp.108-114.
11
Licour, M., Tsatsaris, A. and van't Hof, A. (2013). Ambulance or in-catheterization laboratory
administration of ticagrelor for primary percutaneous coronary intervention for ST-segment
elevation myocardial infarction: Rationale and design of the randomized, double-blind
Administration of Ticagrelor in the cath Lab or in the Ambulance for New ST elevation
myocardial Infarction to open the Coronary artery (ATLANTIC) study. American Heart Journal,
165(4), pp.515-522.
Moore, P., Moore, E. and Moore, F. (2009). Exception from informed consent requirements for
emergency research. Surgery, 145(6), pp.630-635.
Morgans, A. (2010). Waiver of Informed Consent in Prehospital Emergency Health Research in
Australia. Monash Bioethics Review, 29(1), pp.49-64.
Rawbone, R. (2010). Consent: Informed or Not Informed, That is the Question. Research Ethics, 6(4),
pp.115-116.
Sandman, L. and Nordmark, A. (2006). Ethical Conflicts in Prehospital Emergency Care. Nursing
Ethics, 13(6), pp.592-607.
Saver, J., Kidwell, C., Eckstein, M., Ovbiagele, B. and Starkman, S. (2006). Physician-Investigator
Phone Elicitation of Consent in the Field: A Novel Method To Obtain Explicit Informed Consent
For Prehospital Clinical Research. Prehospital Emergency Care, 10(2), pp.182-185.
Snooks, H., Cheung, W., Close, J., Dale, J., Gaze, S., Humphreys, I., Lyons, R., Mason, S., Merali, Y.,
Peconi, J., Phillips, C., Phillips, J., Roberts, S., Russell, I., Sánchez, A., Wani, M., Wells, B. and
Whitfield, R. (2010). Support and Assessment for Fall Emergency Referrals (SAFER 1) trial
protocol. Computerised on-scene decision support for emergency ambulance staff to assess and
plan care for older people who have fallen: evaluation of costs and benefits using a pragmatic
cluster randomised trial. BMC Emergency Medicine, 10(1).
Steg, P., van ‘t Hof, A., Clemmensen, P., Lapostolle, F., Dudek, D., Hamon, M., Cavallini, C., Gordini,
G., Huber, K., Coste, P., Thicoipe, M., Nibbe, L., Steinmetz, J., Ten Berg, J., Eggink, G.,
Zeymer, U., Campo dell' Orto, M., Kanic, V., Deliargyris, E., Day, J., Schuette, D., Hamm, C.
and Goldstein, P. (2013). Design and methods of European Ambulance Acute Coronary
Syndrome Angiography Trial (EUROMAX): An international randomized open-label ambulance
trial of bivalirudin versus standard-of-care anticoagulation in patients with acute ST-segment-
elevation myocardial infarction transferred for primary percutaneous coronary intervention.
American Heart Journal, 166(6), pp.960-967.e6.
Thompson, J. (2003). Ethical challenges of informed consent in prehospital research. CJEM, 5(02),
pp.108-114.
11
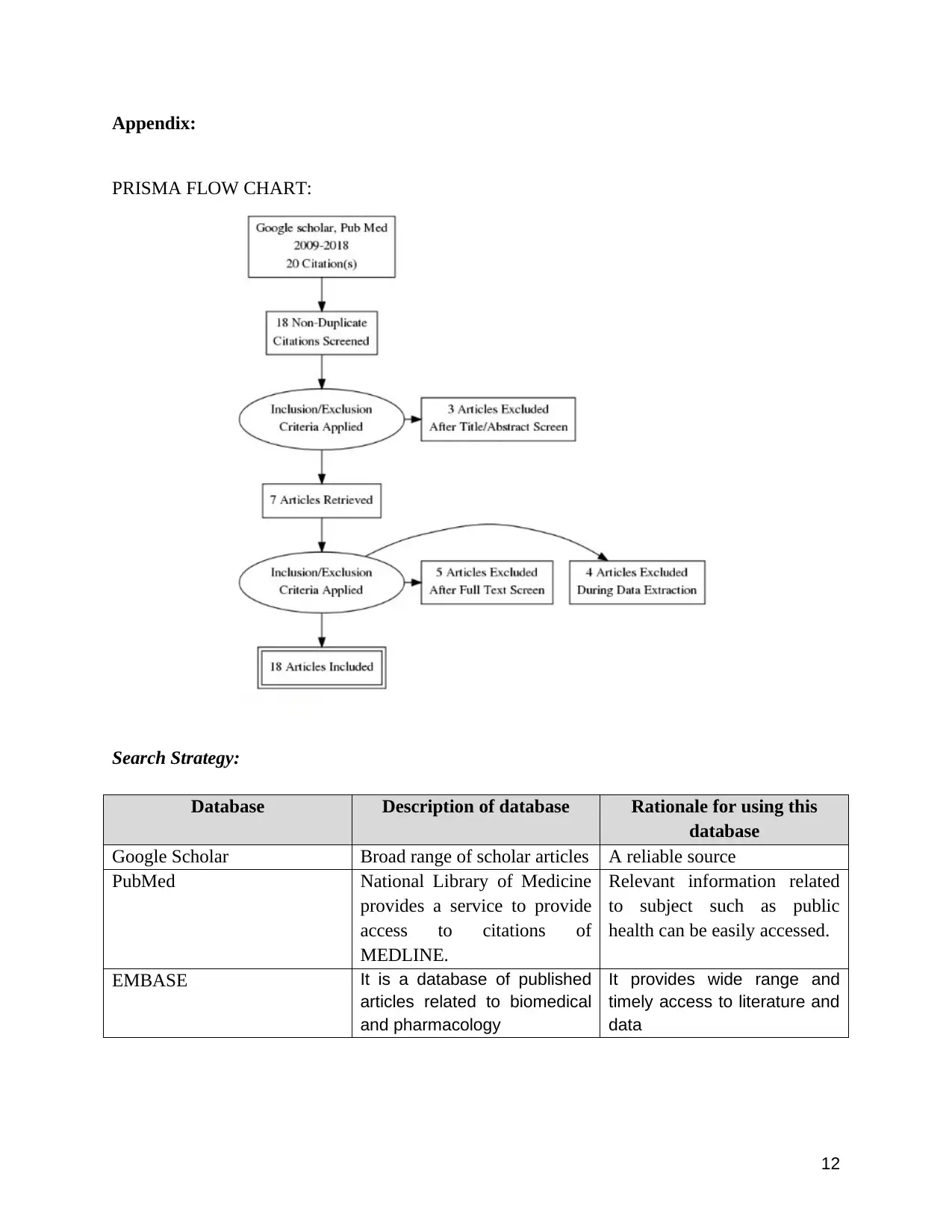
Appendix:
PRISMA FLOW CHART:
Search Strategy:
Database Description of database Rationale for using this
database
Google Scholar Broad range of scholar articles A reliable source
PubMed National Library of Medicine
provides a service to provide
access to citations of
MEDLINE.
Relevant information related
to subject such as public
health can be easily accessed.
EMBASE It is a database of published
articles related to biomedical
and pharmacology
It provides wide range and
timely access to literature and
data
12
PRISMA FLOW CHART:
Search Strategy:
Database Description of database Rationale for using this
database
Google Scholar Broad range of scholar articles A reliable source
PubMed National Library of Medicine
provides a service to provide
access to citations of
MEDLINE.
Relevant information related
to subject such as public
health can be easily accessed.
EMBASE It is a database of published
articles related to biomedical
and pharmacology
It provides wide range and
timely access to literature and
data
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





