Evaluating M-BH33 Drug Efficacy on Mega BCL-2 Protein in Mice
VerifiedAdded on 2022/11/14
|6
|1241
|1
Project
AI Summary
This project investigates the efficacy of the newly invented M-BH33 mimetic drug on Mega BCL-2 protein using a transgenic mouse model. The study's rationale centers on the use of mice in cancer research due to their genomic and physiological similarities to humans. The research aims to create a transgenic mouse model with overexpression of Mega BCL-2 protein, which regulates the mitochondrial apoptotic response, and evaluate the impact of M-BH33 on its expression. The methodology involves a knock-in model, in vitro analysis using HL-60 cells, and in vivo analysis in the transgenic mice. The results indicate the drug's effectiveness, with increased fluorescence in vitro. The study concludes that testing drugs on apoptotic signaling pathways could lead to therapeutic strategies for apoptosis deficiencies, potentially offering new cancer treatments.

Efficacy of M-BH33, the new BH3 mimetic drug on Mega BCL-2 protein
Rationale
The utilisation of mice in cancer research has been significant owing to its similarity in
genomic, anatomical, molecular and physiological characteristics of cancer biology with
humans (Tratar et al., 2018). In addition, mice reproduce rapidly, easy to maintain in the
laboratory and provides the capability to assess the etiology of the disease at a low cost.
Despite conventionally immune-deficient mice with grafted tumours have been utilised
earlier, the emergence of transgenic mouse models have been potential over the past three
decades. In 2007, the Federation of European Laboratory Animal Science Associations
specified that transgenic mice are those that are involved with spontaneous and chemically
induced mutations. Furthermore, the National Institute of Health and the National Cancer
Institute specifically define transgenic mouse as those where the DNA from the genome of
mouse or other species is incorporated into the genome of the mouse model. They are also
collectively referred as germ-line genetically engineered mouse models (GEMM).
Currently, the focus has shifted from studying spontaneous and chemically-induced mouse
models to study the expression and function of genes via knockin and knockout approach
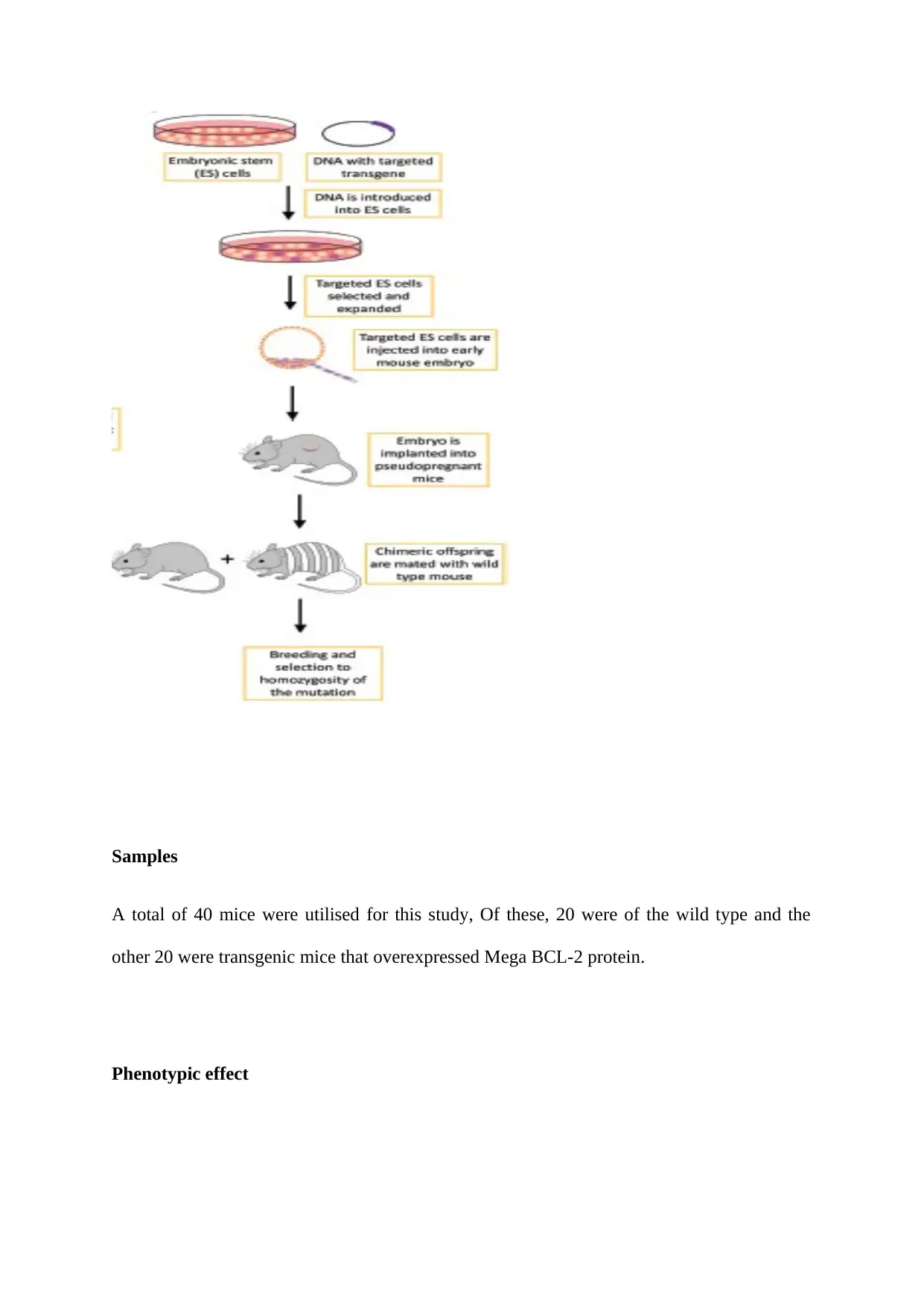
(Kersten et al., 2016). As such, various approaches such as i) retroviral infection of mouse
embryos during development, ii) standard transgene-based method via microinjection od
DNA constructs into the nucleus of fertilised oocytes of mouse iii) and gene-targeted
transgene-based manipulation of the embryonic stem cells at a particular loci leading to a
primary loss of function mutations (Fig 1). Transgenic mice can be categorised into two
groups based on the loss (knockout model) or gain (knock-in model) of the functional genes.
The loss of function provides information about the function of a normal gene. Two types of
knockout models are frequently utilised, i) constitutive where the gene expression is
Rationale
The utilisation of mice in cancer research has been significant owing to its similarity in
genomic, anatomical, molecular and physiological characteristics of cancer biology with
humans (Tratar et al., 2018). In addition, mice reproduce rapidly, easy to maintain in the
laboratory and provides the capability to assess the etiology of the disease at a low cost.
Despite conventionally immune-deficient mice with grafted tumours have been utilised
earlier, the emergence of transgenic mouse models have been potential over the past three
decades. In 2007, the Federation of European Laboratory Animal Science Associations
specified that transgenic mice are those that are involved with spontaneous and chemically
induced mutations. Furthermore, the National Institute of Health and the National Cancer
Institute specifically define transgenic mouse as those where the DNA from the genome of
mouse or other species is incorporated into the genome of the mouse model. They are also
collectively referred as germ-line genetically engineered mouse models (GEMM).
Currently, the focus has shifted from studying spontaneous and chemically-induced mouse
models to study the expression and function of genes via knockin and knockout approach
(Kersten et al., 2016). As such, various approaches such as i) retroviral infection of mouse
embryos during development, ii) standard transgene-based method via microinjection od
DNA constructs into the nucleus of fertilised oocytes of mouse iii) and gene-targeted
transgene-based manipulation of the embryonic stem cells at a particular loci leading to a
primary loss of function mutations (Fig 1). Transgenic mice can be categorised into two
groups based on the loss (knockout model) or gain (knock-in model) of the functional genes.
The loss of function provides information about the function of a normal gene. Two types of
knockout models are frequently utilised, i) constitutive where the gene expression is
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

permanently inactivated in each cell of the mouse and ii) conditional where the gene
expression undergoes inducible inactivation directly affective the target.
On the contrary, the gain of function reveals information about overexpression of oncogenes
and its effect in carcinogenesis. Four different knock-in models are used, i) constitutive
random insertion model, knock-in permissive locus model, conditional knock-in model and
reporter knock-in model. In addition to the aforementioned transgenic mouse models, new
models have such as non-germline genetically engineered mouse models and alternative
DNA modification techniques such as transposon based insertion mutagenesis, RNA
interference, engineered nucleases and CRISPR/Cas9 system have emerged in studying the
expression of oncogenes.
Mega BCL-2 protein regulates the mitochondrial apoptotic response, thereby possessing the
ability to suppress apoptosis leading to the development of cancer (Hata et al., 2015).
Furthermore, BH3 mimetic drugs offer a new chemotherapy for cancer by binding to BCL-2
family members and neutralizing the anti-apoptotic effect (Merino et al.,2018). Henceforth,
in this study, a transgenic mouse model will be created with the overexpression of Mega
BCL-2 protein. Additionally, M-BH33, a new BH3 mimetic will be inventedand administered
to evaluate its impact on the expression of Mega BCL-2 protein in the transgenic mouse
model.
Hypothesis
Overexpression of Mega BCL-2 protein in a transgenic cell could suppress apoptosis
and stimulate carcinogenesis
The new drug M-BH33 inhibits the anti-apoptotic protein Mega BCL-2 from
proliferation
The drug leads to overexpression of BH3 pro-apoptotic protein
expression undergoes inducible inactivation directly affective the target.
On the contrary, the gain of function reveals information about overexpression of oncogenes
and its effect in carcinogenesis. Four different knock-in models are used, i) constitutive
random insertion model, knock-in permissive locus model, conditional knock-in model and
reporter knock-in model. In addition to the aforementioned transgenic mouse models, new
models have such as non-germline genetically engineered mouse models and alternative
DNA modification techniques such as transposon based insertion mutagenesis, RNA
interference, engineered nucleases and CRISPR/Cas9 system have emerged in studying the
expression of oncogenes.
Mega BCL-2 protein regulates the mitochondrial apoptotic response, thereby possessing the
ability to suppress apoptosis leading to the development of cancer (Hata et al., 2015).
Furthermore, BH3 mimetic drugs offer a new chemotherapy for cancer by binding to BCL-2
family members and neutralizing the anti-apoptotic effect (Merino et al.,2018). Henceforth,
in this study, a transgenic mouse model will be created with the overexpression of Mega
BCL-2 protein. Additionally, M-BH33, a new BH3 mimetic will be inventedand administered
to evaluate its impact on the expression of Mega BCL-2 protein in the transgenic mouse
model.
Hypothesis
Overexpression of Mega BCL-2 protein in a transgenic cell could suppress apoptosis
and stimulate carcinogenesis
The new drug M-BH33 inhibits the anti-apoptotic protein Mega BCL-2 from
proliferation
The drug leads to overexpression of BH3 pro-apoptotic protein

Aim and objectives
The overarching aims of this study are:
To create a transgenic mouse model with the overexpression of Mega BCL-2 protein
Investigating the efficiency of expression in transgenic mouse model to promote
cancer phenotype
To analyse the efficacy of the newly invented M-BH33 mimetic drugs in vitro and in
vivo
Methodology
The knock-in (gain-of-function) model is used to test the drug efficacy on the expression of
Mega BCL-2 protein.
Design of a transgenic mouse model
The overarching aims of this study are:
To create a transgenic mouse model with the overexpression of Mega BCL-2 protein
Investigating the efficiency of expression in transgenic mouse model to promote
cancer phenotype
To analyse the efficacy of the newly invented M-BH33 mimetic drugs in vitro and in
vivo
Methodology
The knock-in (gain-of-function) model is used to test the drug efficacy on the expression of
Mega BCL-2 protein.
Design of a transgenic mouse model
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Samples
A total of 40 mice were utilised for this study, Of these, 20 were of the wild type and the
other 20 were transgenic mice that overexpressed Mega BCL-2 protein.
Phenotypic effect
A total of 40 mice were utilised for this study, Of these, 20 were of the wild type and the
other 20 were transgenic mice that overexpressed Mega BCL-2 protein.
Phenotypic effect
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

The phenotypic effect in the animal was measured using the difference in the weight of the
animal before and after the incorporation of Mega BCL-2 protein thereby providing a clear
indication of the health. In addition the use of survival graph denoted the mortality rate.
In vitro analysis of drug efficacy
The efficacy of M-BH33 drug was tested using Caspase 3 activity in the wild type and
transgenic human leukaemia (HL-60) cells possessing the overexpressed Mega BCL-2-2
protein. Each assay had a control (untreated). The fluorescence assay revealed that the
cleavage of the caspase substrate DEVD from AFC (7-amino-4-trifluoreomethylcoumarin) in
the caspase recognition sequence due to the effect of M-BH33 mimetic. The fluorescence
emitted is directly proportional to the drug efficacy.
In vivo drug efficacy
Based on the results obtained in vitro, the drug was tested for its efficacy in vivo in a
transgenic mouse model that overexpressed Mega BCL-2-2 protein. 6 genetically similar
mice were injected with tumour cells subcutaneously. One pair acted as the control without
M-BH33 mimetic drug while the other two pairs were the experimental groups and were
administered with BH3 mimetic drugs.
Results and Discussions
The subcutaneous injection of mice with cancer cells resulted in the formation of tumour in
the mice. The protein Mega BCL-2-2 was administered to evaluate the development of cancer
via weight measurement and mortality rate. The drug M-BH33 mimetic was effective in vitro
animal before and after the incorporation of Mega BCL-2 protein thereby providing a clear
indication of the health. In addition the use of survival graph denoted the mortality rate.
In vitro analysis of drug efficacy
The efficacy of M-BH33 drug was tested using Caspase 3 activity in the wild type and
transgenic human leukaemia (HL-60) cells possessing the overexpressed Mega BCL-2-2
protein. Each assay had a control (untreated). The fluorescence assay revealed that the
cleavage of the caspase substrate DEVD from AFC (7-amino-4-trifluoreomethylcoumarin) in
the caspase recognition sequence due to the effect of M-BH33 mimetic. The fluorescence
emitted is directly proportional to the drug efficacy.
In vivo drug efficacy
Based on the results obtained in vitro, the drug was tested for its efficacy in vivo in a
transgenic mouse model that overexpressed Mega BCL-2-2 protein. 6 genetically similar
mice were injected with tumour cells subcutaneously. One pair acted as the control without
M-BH33 mimetic drug while the other two pairs were the experimental groups and were
administered with BH3 mimetic drugs.
Results and Discussions
The subcutaneous injection of mice with cancer cells resulted in the formation of tumour in
the mice. The protein Mega BCL-2-2 was administered to evaluate the development of cancer
via weight measurement and mortality rate. The drug M-BH33 mimetic was effective in vitro

and it was evident with increased fluorescence. One approach to deliver the results faster is to
utilise the principle of bioluminescence to differentiate between tumour and normal cells.
Conclusion
The study examined the effects of M-BH33,, the newly invented BH3 mimetic drug using
transgenic mouse that overexpressed Mega BCL-2-2 protein. Testing the efficacy of drugs on
proteins involved in the apoptotic signalling pathways will lead to develop therapeutic
strategies for apoptosis deficiencies thereby acting as a potential chemotherapy for cancer.
References
Tratar U. L., Horvat S., Cemazar M. (2018). Transgenic mouse models in cancer research.
Frontiers in Oncology, https://doi.org/10.3389/fonc.2018.00268
Kersten K., de Vesser K. E., van Mileten burg M. H., Jonkers Jos. (2016). Genetically
engineered mouse models in oncology research and cancer medicine. EMBO
Molecular Medicine. Vol 9 (2). 137-153.
Hata A. N., Engelman J. A., Faber A. C. (2015). The BCL-2 family: key mediators of the
apoptotic response to targeted anti-cancer therapeutics. Cancer Discovery. Vol 5 (5).
475-487
Merino D., Kelly G. L., Lessene G., Wei A. H., Roberts A. W., Strasser A. (2018). BH3-
mimetic drugs: biazing the trial for new cancer medicines. Cancer Cell. Vol 34 (6).
879-891
utilise the principle of bioluminescence to differentiate between tumour and normal cells.
Conclusion
The study examined the effects of M-BH33,, the newly invented BH3 mimetic drug using
transgenic mouse that overexpressed Mega BCL-2-2 protein. Testing the efficacy of drugs on
proteins involved in the apoptotic signalling pathways will lead to develop therapeutic
strategies for apoptosis deficiencies thereby acting as a potential chemotherapy for cancer.
References
Tratar U. L., Horvat S., Cemazar M. (2018). Transgenic mouse models in cancer research.
Frontiers in Oncology, https://doi.org/10.3389/fonc.2018.00268
Kersten K., de Vesser K. E., van Mileten burg M. H., Jonkers Jos. (2016). Genetically
engineered mouse models in oncology research and cancer medicine. EMBO
Molecular Medicine. Vol 9 (2). 137-153.
Hata A. N., Engelman J. A., Faber A. C. (2015). The BCL-2 family: key mediators of the
apoptotic response to targeted anti-cancer therapeutics. Cancer Discovery. Vol 5 (5).
475-487
Merino D., Kelly G. L., Lessene G., Wei A. H., Roberts A. W., Strasser A. (2018). BH3-
mimetic drugs: biazing the trial for new cancer medicines. Cancer Cell. Vol 34 (6).
879-891
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.