MAE 204: Fall 2019 Homework Assignment #7 on Thermodynamics Problems
VerifiedAdded on 2022/10/04
|5
|663
|18
Homework Assignment
AI Summary
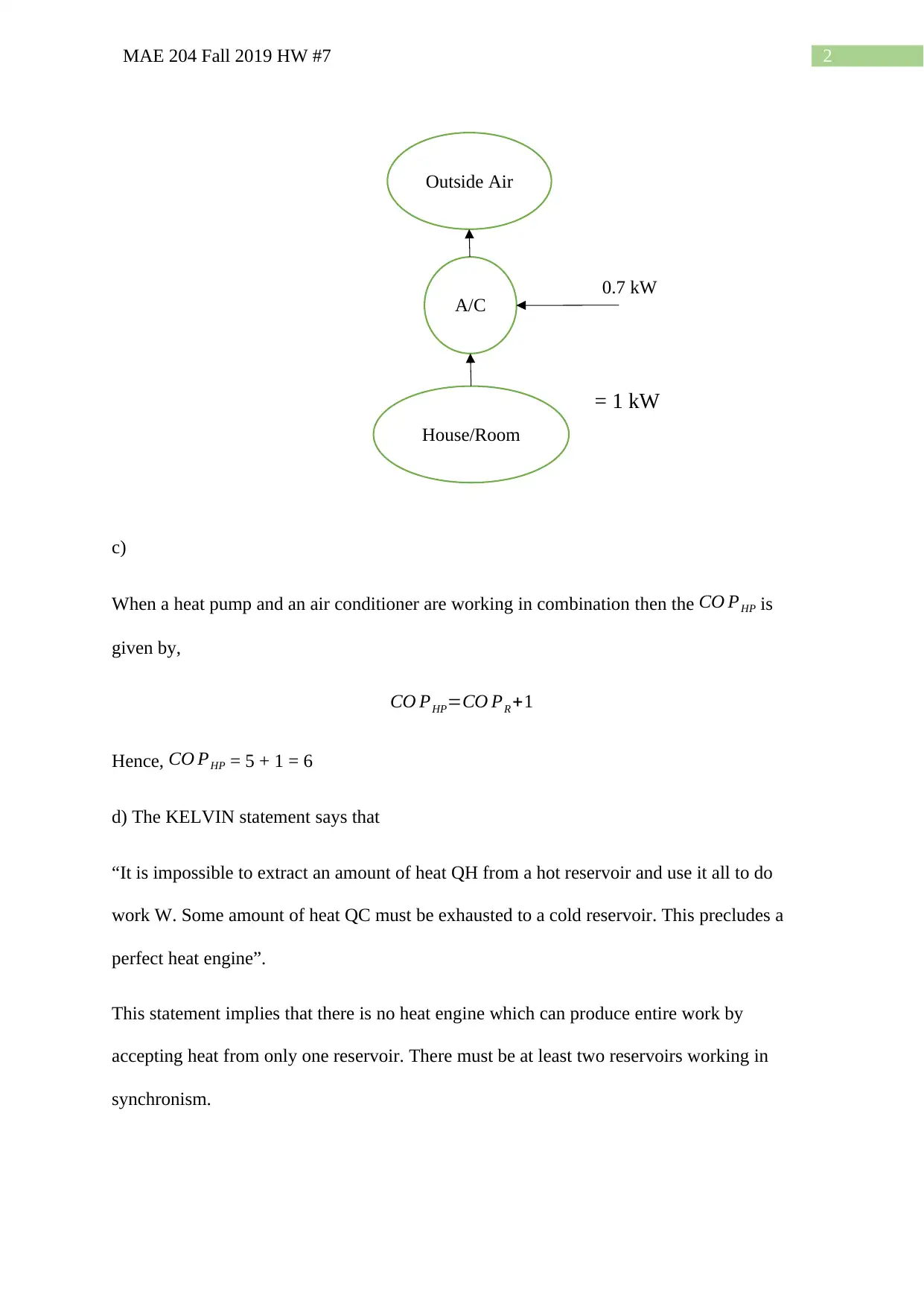
This document presents the solutions to Homework #7 for MAE 204, a mechanical engineering course, from Fall 2019. The assignment covers several key concepts in thermodynamics. Problem 1 involves calculating work produced and heat rejected by a heat engine, determining the coefficient of performance (COP) of an air conditioner, analyzing a combined air conditioner/heat pump system, and identifying the Kelvin statement. Problem 2 focuses on calculating the total power output of a steady-flow turbine given inlet and outlet conditions, mass flow rate, and heat loss. Problem 3 addresses the calculation of the final temperature and mass of air in a tank, and determining the heat transfer involved. The solutions demonstrate the application of thermodynamic principles, including energy conservation and the use of steam tables. The document provides step-by-step solutions to the problems, including calculations and explanations.
1 out of 5





![[object Object]](/_next/static/media/star-bottom.7253800d.svg)