University Report: Partnerships for Probiotics Manufacturing Analysis
VerifiedAdded on 2023/06/06
|44
|11668
|233
Report
AI Summary
This report, titled "Partnerships for Probiotics Manufacturing," delves into the strategic alliances within the probiotics industry, focusing on identifying optimal companies for outsourcing manufacturing. The research encompasses a comprehensive literature review of management tools like Total Quality Management (TQM), Benchmarking, SWOT analysis, Business Model Canvas, and Partnership relationship management. The methodology involves evaluating companies based on fermentation processes, equipment scales, downstream processing, cost-effectiveness, and quality systems (cGMP). The report analyzes key selection criteria, including external contracts, equipment scale, location, and quality systems, alongside the importance and advantages of partnerships. It also includes a SWOT analysis of partnerships and factors for partner selection, such as regulatory bodies, pilot plants, experienced staff, and track records. Several companies are proposed for potential partnerships, with detailed assessments of their facilities, skills, previous partnerships, and licensing status. The report concludes with recommendations for successful partnerships, aiming to enhance production, promote animal health, and drive industry growth. The findings are valuable for companies looking to optimize their probiotic manufacturing processes and leverage strategic alliances for competitive advantage.

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING1
FINAL PROJECT REPORT
Name
Course
Professor
University
City/state
Date
FINAL PROJECT REPORT
Name
Course
Professor
University
City/state
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING2
Table of Contents
1. Introduction.......................................................................................................................................4
2. An Objective of the Research............................................................................................................4
3. The scope of the Research.................................................................................................................5
4. Significance of the research..............................................................................................................5
5. Definition of Probiotic and the Classification..................................................................................5
6. Literature review...............................................................................................................................7
6.1. Identification of management tools..........................................................................................7
6.1.1. Total quality management (TQM).......................................................................................7
6.1.2. Benchmarking......................................................................................................................7
6.1.3. SWOT analysis....................................................................................................................8
6.1.4. The business model canvas..................................................................................................9
6.1.5. Partnerships.........................................................................................................................9
7. The Methodology of the Work........................................................................................................10
7.1. The manufacturing of the probiotics......................................................................................10
7.2. Selection criteria for each company.......................................................................................10
8. Results and discussion.....................................................................................................................11
8.1. Fermentation process used......................................................................................................11
8.2. Fermentation methods used by the companies......................................................................11
8.3. Drying.......................................................................................................................................12
9. Key selection criteria.......................................................................................................................13
9.1. External contracts/toll manufacturing...................................................................................13
9.2. The scale of fermentation equipment.....................................................................................14
9.3. Downstream processing equipment (centrifuges, dryers).....................................................15
10. Location of the Company and Cost-effectiveness of probiotics................................................16
10.1. Results of th Study...............................................................................................................16
10.2. Quality systems (cGMP)......................................................................................................17
11. Importance and advantages partnerships and joint ventures..................................................18
12. SWOT analysis of working in a partnership with other company...........................................20
12.1. Strengths...............................................................................................................................20
12.2. Weaknesses...........................................................................................................................21
12.3. Opportunities.......................................................................................................................22
12.4. Threats..................................................................................................................................24
Table of Contents
1. Introduction.......................................................................................................................................4
2. An Objective of the Research............................................................................................................4
3. The scope of the Research.................................................................................................................5
4. Significance of the research..............................................................................................................5
5. Definition of Probiotic and the Classification..................................................................................5
6. Literature review...............................................................................................................................7
6.1. Identification of management tools..........................................................................................7
6.1.1. Total quality management (TQM).......................................................................................7
6.1.2. Benchmarking......................................................................................................................7
6.1.3. SWOT analysis....................................................................................................................8
6.1.4. The business model canvas..................................................................................................9
6.1.5. Partnerships.........................................................................................................................9
7. The Methodology of the Work........................................................................................................10
7.1. The manufacturing of the probiotics......................................................................................10
7.2. Selection criteria for each company.......................................................................................10
8. Results and discussion.....................................................................................................................11
8.1. Fermentation process used......................................................................................................11
8.2. Fermentation methods used by the companies......................................................................11
8.3. Drying.......................................................................................................................................12
9. Key selection criteria.......................................................................................................................13
9.1. External contracts/toll manufacturing...................................................................................13
9.2. The scale of fermentation equipment.....................................................................................14
9.3. Downstream processing equipment (centrifuges, dryers).....................................................15
10. Location of the Company and Cost-effectiveness of probiotics................................................16
10.1. Results of th Study...............................................................................................................16
10.2. Quality systems (cGMP)......................................................................................................17
11. Importance and advantages partnerships and joint ventures..................................................18
12. SWOT analysis of working in a partnership with other company...........................................20
12.1. Strengths...............................................................................................................................20
12.2. Weaknesses...........................................................................................................................21
12.3. Opportunities.......................................................................................................................22
12.4. Threats..................................................................................................................................24

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING3
13. Factors to Consider when Looking for Partnership or Joint Venture or Partners................25
14. Regulatory bodies........................................................................................................................28
15. Other Partner Selection Criteria................................................................................................29
15.1. Pilot plant.............................................................................................................................29
15.2. Experienced staff.................................................................................................................30
15.3. Network................................................................................................................................30
15.4. Track record........................................................................................................................30
15.5. Accredited.............................................................................................................................30
15.6. Partners................................................................................................................................31
16. Proposed Companies for Partnership or Joint Venture...........................................................31
16.1. Location................................................................................................................................31
16.2. Core business........................................................................................................................32
16.3. Facilities and equipment......................................................................................................32
16.4. Skills and experience...........................................................................................................32
16.5. Previous partnerships..........................................................................................................32
16.6. Quality systems....................................................................................................................32
16.7. Licensing status....................................................................................................................33
16.8. Other reasons why they are the best partners...................................................................33
17. Proposed Companies...................................................................................................................33
18. Conclusion....................................................................................................................................36
References................................................................................................................................................37
13. Factors to Consider when Looking for Partnership or Joint Venture or Partners................25
14. Regulatory bodies........................................................................................................................28
15. Other Partner Selection Criteria................................................................................................29
15.1. Pilot plant.............................................................................................................................29
15.2. Experienced staff.................................................................................................................30
15.3. Network................................................................................................................................30
15.4. Track record........................................................................................................................30
15.5. Accredited.............................................................................................................................30
15.6. Partners................................................................................................................................31
16. Proposed Companies for Partnership or Joint Venture...........................................................31
16.1. Location................................................................................................................................31
16.2. Core business........................................................................................................................32
16.3. Facilities and equipment......................................................................................................32
16.4. Skills and experience...........................................................................................................32
16.5. Previous partnerships..........................................................................................................32
16.6. Quality systems....................................................................................................................32
16.7. Licensing status....................................................................................................................33
16.8. Other reasons why they are the best partners...................................................................33
17. Proposed Companies...................................................................................................................33
18. Conclusion....................................................................................................................................36
References................................................................................................................................................37
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING4
1. Introduction
Generally, the world population is expected to be almost more than 10 billion in 2040.
Due to changes in the economic growth of the world, there has been a big demand to
manufacture adequate feed needed by the livestock. This has raised a big concern, therefore,
coming up with technologized mechanisms for manufacturing enough feed for the livestock
regardless of the limited resources.
However, the livestock sector has been growing rapidly as compared to the other sectors
of agriculture. It accounts for almost 50% of the total value within the field of agriculture.
Generally, the increasing human population will increase the livestock numbers. Livestock
production will be able to support millions of people by providing adequate food and other
agricultural related materials (Ruiz-Moyano, et. al., 2011). Generally, livestock will be able to
provide an income for instance in marginalized areas and it will also serve as the key factor
which will assist in fighting the issues of poverty. Increased livestock production will also
improve soil fertility because of manure and also boost fuel that is made from livestock waste
used in biogas plants. Most of the livestock systems all over the world are gearing towards the
improvement of supply of the number of the livestock. This has influenced the formation of the
livestock welfares issues that target to manufacture adequate feed for the existence of the
livestock. Because of increase demand for livestock supply, stakeholders involved with livestock
production are now focusing on probiotics (Ruggles, 2009).
2. An Objective of the Research
The objective of the research was to identify the best company for outsourcing the
manufacture of probiotics. The probiotics (the direct fed microbes) are rapidly used nowadays as
the common livestock feeds. They are the commonly used feeds supplements in the field of
1. Introduction
Generally, the world population is expected to be almost more than 10 billion in 2040.
Due to changes in the economic growth of the world, there has been a big demand to
manufacture adequate feed needed by the livestock. This has raised a big concern, therefore,
coming up with technologized mechanisms for manufacturing enough feed for the livestock
regardless of the limited resources.
However, the livestock sector has been growing rapidly as compared to the other sectors
of agriculture. It accounts for almost 50% of the total value within the field of agriculture.
Generally, the increasing human population will increase the livestock numbers. Livestock
production will be able to support millions of people by providing adequate food and other
agricultural related materials (Ruiz-Moyano, et. al., 2011). Generally, livestock will be able to
provide an income for instance in marginalized areas and it will also serve as the key factor
which will assist in fighting the issues of poverty. Increased livestock production will also
improve soil fertility because of manure and also boost fuel that is made from livestock waste
used in biogas plants. Most of the livestock systems all over the world are gearing towards the
improvement of supply of the number of the livestock. This has influenced the formation of the
livestock welfares issues that target to manufacture adequate feed for the existence of the
livestock. Because of increase demand for livestock supply, stakeholders involved with livestock
production are now focusing on probiotics (Ruggles, 2009).
2. An Objective of the Research
The objective of the research was to identify the best company for outsourcing the
manufacture of probiotics. The probiotics (the direct fed microbes) are rapidly used nowadays as
the common livestock feeds. They are the commonly used feeds supplements in the field of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING5
agriculture that have been used as the substitute of the Antibiotic Growth Promoters (Ruiz-
Moyano, et. al., 2011). The main aim to use the probiotics in livestock is to increase the
production level and enhance the issue of growth and development in livestock. It also assists in
controlling the entry of the pathogens to the livestock thus providing a better life for the animal
to survive in any environment. These food additives will enhance the efficiency of the livestock
since there will be adequate food for livestock survival.
3. The scope of the Research
The research was only limited to the production of the probiotics and the use of specified
criteria in designing the probiotics. The criteria were considered when selecting appropriate
company that had a good quality for manufacturing probiotics (Kudełka, 2010).
4. Significance of the research
This research project is important as it involved ensuring strategies that companies apply
in the manufacture of probiotics. The best partner company to be selected is key in the
development of quality probiotic products to enhance consumer satisfaction and promote the
health of the animals The significance of this research is to curb the above outlined problems
regarding manufacture of probiotics in order to prevent pathogenic bacteria in animals and
promote their health (Rigby & Bilodeau, 2011).
5. Definition of Probiotic and the Classification
Different researchers have defined the term probiotic as a dietary or dairy food
supplement containing live yeasts and bacteria and adds or replaces the beneficial bacterial
usually found in the digestive system or gastrointestinal duct. This gives a description of the
microbial which help in intestinal microbial balance within an organism. The microbial are living
agriculture that have been used as the substitute of the Antibiotic Growth Promoters (Ruiz-
Moyano, et. al., 2011). The main aim to use the probiotics in livestock is to increase the
production level and enhance the issue of growth and development in livestock. It also assists in
controlling the entry of the pathogens to the livestock thus providing a better life for the animal
to survive in any environment. These food additives will enhance the efficiency of the livestock
since there will be adequate food for livestock survival.
3. The scope of the Research
The research was only limited to the production of the probiotics and the use of specified
criteria in designing the probiotics. The criteria were considered when selecting appropriate
company that had a good quality for manufacturing probiotics (Kudełka, 2010).
4. Significance of the research
This research project is important as it involved ensuring strategies that companies apply
in the manufacture of probiotics. The best partner company to be selected is key in the
development of quality probiotic products to enhance consumer satisfaction and promote the
health of the animals The significance of this research is to curb the above outlined problems
regarding manufacture of probiotics in order to prevent pathogenic bacteria in animals and
promote their health (Rigby & Bilodeau, 2011).
5. Definition of Probiotic and the Classification
Different researchers have defined the term probiotic as a dietary or dairy food
supplement containing live yeasts and bacteria and adds or replaces the beneficial bacterial
usually found in the digestive system or gastrointestinal duct. This gives a description of the
microbial which help in intestinal microbial balance within an organism. The microbial are living

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING6
organisms and they improve the intestinal balance of the host animal thus enhancing growth. We
have a number of microbial used in different companies that have been classified as follows:
Bacterial and the non-bacterial probiotics: they include use of micro-organisms such as
the bacteria (Sanders, 2008). The companies use different species of the bacteria to manufacture
the probiotics. Some of the species are categorized as the Lactobacillus and the Candida
pintolopesii.
Spore-forming and the non-spore forming probiotics: although the non-spore forming
probiotics predominates over the spore-forming probiotics, in most of the companies you find
that the most used is the spore-forming probiotics. This is because it has good quality and
therefore giving adequate feeds for the livestock survival.
Autochthonous probiotics and the Allochthonous probiotics: some of the companies uses
bacteria which are absent in the gut of the animals (Sanders, 2008). The commonly used are the
allochthonous one with the example of the yeast. The other section of the microorganism used is
autochthonous which includes the lactobacillus bacteria.
Multispecies and the single species probiotics used by the companies: the microbial
products of the probiotics usually vary from the single production all the way to the multi-
production in all the companies. Most companies use the microbial that will provide adequate
products such as multispecies while the small company may prefer to use the single species
(Sanders, 2008). They can be used in small production and also in large production depending
on the choice of each company.
organisms and they improve the intestinal balance of the host animal thus enhancing growth. We
have a number of microbial used in different companies that have been classified as follows:
Bacterial and the non-bacterial probiotics: they include use of micro-organisms such as
the bacteria (Sanders, 2008). The companies use different species of the bacteria to manufacture
the probiotics. Some of the species are categorized as the Lactobacillus and the Candida
pintolopesii.
Spore-forming and the non-spore forming probiotics: although the non-spore forming
probiotics predominates over the spore-forming probiotics, in most of the companies you find
that the most used is the spore-forming probiotics. This is because it has good quality and
therefore giving adequate feeds for the livestock survival.
Autochthonous probiotics and the Allochthonous probiotics: some of the companies uses
bacteria which are absent in the gut of the animals (Sanders, 2008). The commonly used are the
allochthonous one with the example of the yeast. The other section of the microorganism used is
autochthonous which includes the lactobacillus bacteria.
Multispecies and the single species probiotics used by the companies: the microbial
products of the probiotics usually vary from the single production all the way to the multi-
production in all the companies. Most companies use the microbial that will provide adequate
products such as multispecies while the small company may prefer to use the single species
(Sanders, 2008). They can be used in small production and also in large production depending
on the choice of each company.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
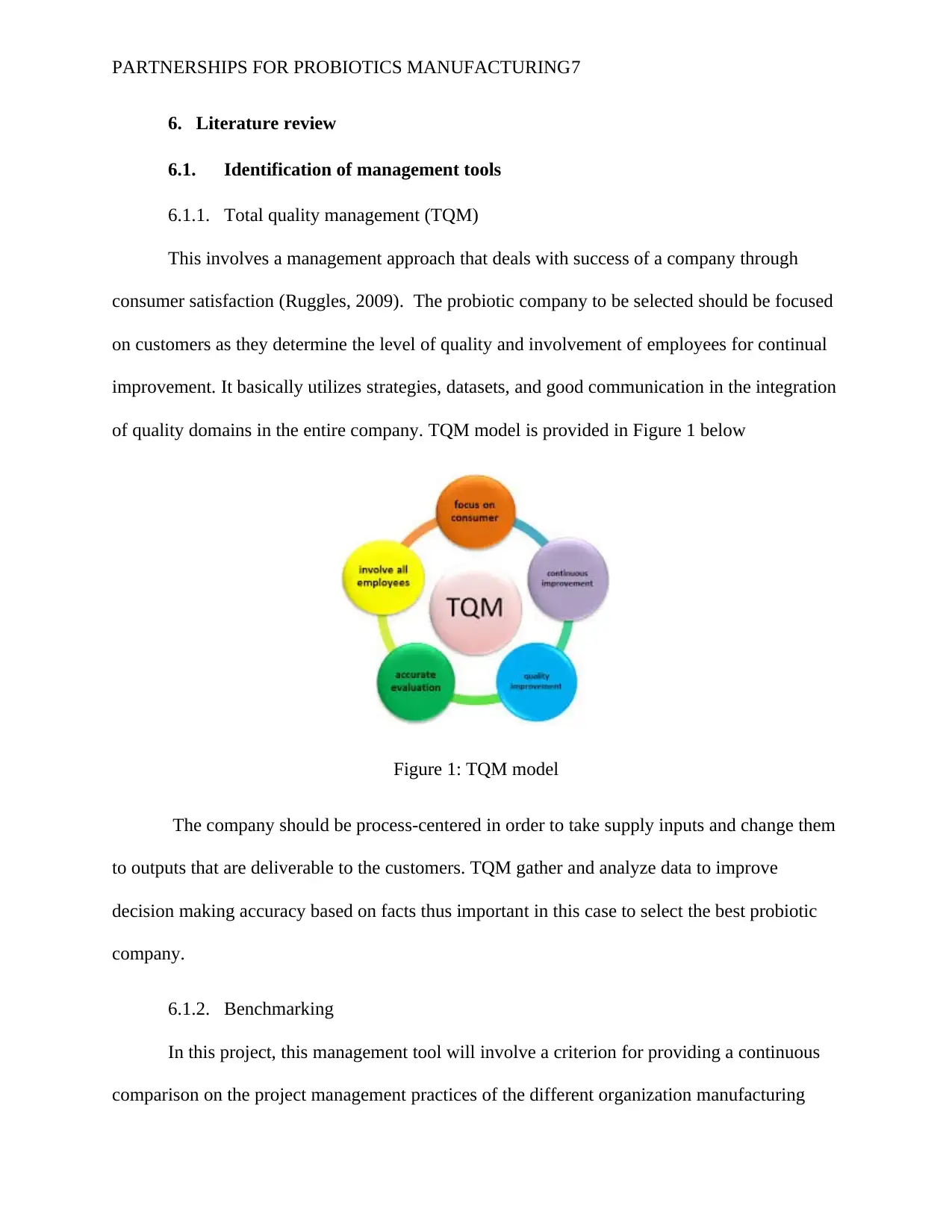
PARTNERSHIPS FOR PROBIOTICS MANUFACTURING7
6. Literature review
6.1. Identification of management tools
6.1.1. Total quality management (TQM)
This involves a management approach that deals with success of a company through
consumer satisfaction (Ruggles, 2009). The probiotic company to be selected should be focused
on customers as they determine the level of quality and involvement of employees for continual
improvement. It basically utilizes strategies, datasets, and good communication in the integration
of quality domains in the entire company. TQM model is provided in Figure 1 below
Figure 1: TQM model
The company should be process-centered in order to take supply inputs and change them
to outputs that are deliverable to the customers. TQM gather and analyze data to improve
decision making accuracy based on facts thus important in this case to select the best probiotic
company.
6.1.2. Benchmarking
In this project, this management tool will involve a criterion for providing a continuous
comparison on the project management practices of the different organization manufacturing
6. Literature review
6.1. Identification of management tools
6.1.1. Total quality management (TQM)
This involves a management approach that deals with success of a company through
consumer satisfaction (Ruggles, 2009). The probiotic company to be selected should be focused
on customers as they determine the level of quality and involvement of employees for continual
improvement. It basically utilizes strategies, datasets, and good communication in the integration
of quality domains in the entire company. TQM model is provided in Figure 1 below
Figure 1: TQM model
The company should be process-centered in order to take supply inputs and change them
to outputs that are deliverable to the customers. TQM gather and analyze data to improve
decision making accuracy based on facts thus important in this case to select the best probiotic
company.
6.1.2. Benchmarking
In this project, this management tool will involve a criterion for providing a continuous
comparison on the project management practices of the different organization manufacturing
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
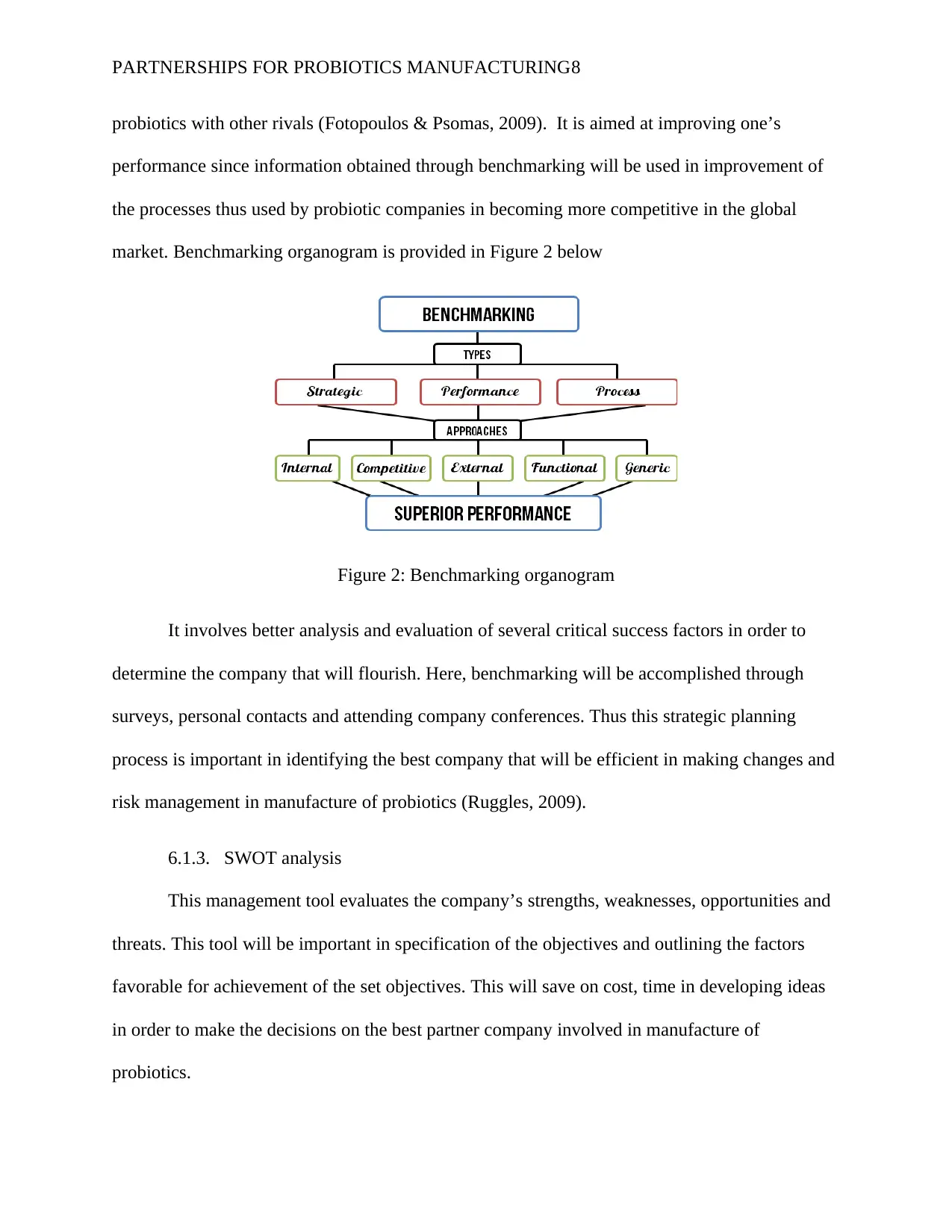
PARTNERSHIPS FOR PROBIOTICS MANUFACTURING8
probiotics with other rivals (Fotopoulos & Psomas, 2009). It is aimed at improving one’s
performance since information obtained through benchmarking will be used in improvement of
the processes thus used by probiotic companies in becoming more competitive in the global
market. Benchmarking organogram is provided in Figure 2 below
Figure 2: Benchmarking organogram
It involves better analysis and evaluation of several critical success factors in order to
determine the company that will flourish. Here, benchmarking will be accomplished through
surveys, personal contacts and attending company conferences. Thus this strategic planning
process is important in identifying the best company that will be efficient in making changes and
risk management in manufacture of probiotics (Ruggles, 2009).
6.1.3. SWOT analysis
This management tool evaluates the company’s strengths, weaknesses, opportunities and
threats. This tool will be important in specification of the objectives and outlining the factors
favorable for achievement of the set objectives. This will save on cost, time in developing ideas
in order to make the decisions on the best partner company involved in manufacture of
probiotics.
probiotics with other rivals (Fotopoulos & Psomas, 2009). It is aimed at improving one’s
performance since information obtained through benchmarking will be used in improvement of
the processes thus used by probiotic companies in becoming more competitive in the global
market. Benchmarking organogram is provided in Figure 2 below
Figure 2: Benchmarking organogram
It involves better analysis and evaluation of several critical success factors in order to
determine the company that will flourish. Here, benchmarking will be accomplished through
surveys, personal contacts and attending company conferences. Thus this strategic planning
process is important in identifying the best company that will be efficient in making changes and
risk management in manufacture of probiotics (Ruggles, 2009).
6.1.3. SWOT analysis
This management tool evaluates the company’s strengths, weaknesses, opportunities and
threats. This tool will be important in specification of the objectives and outlining the factors
favorable for achievement of the set objectives. This will save on cost, time in developing ideas
in order to make the decisions on the best partner company involved in manufacture of
probiotics.

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING9
6.1.4. The business model canvas
This involves development of new modification of the available company models. The
best partner company for the manufacture of probiotics will be provide the visual chart that
consist of elements of the company product value, customers, transportation means and financial
resources. (Fotopoulos & Psomas, 2009). This model will also involve how a company will
generate revenue streams such as asset sale, leasing and advertising through media. The business
Model canvas ought to be printed on the company board in order to identify the best partner
(Rigby & Bilodeau, 2011).
6.1.5. Partnerships
Partnership relationship management tool involves the existing processes and strategies
that a company utilize in directing the operations with partners who make product sales. This
approach includes partner portal, consumer computer databases that ensure various companies
and partners in management of sales metrics, prices and inventories of the manufacture product
(Fotopoulos & Psomas, 2009). Probiotic manufacturing companies should trust on partner
companies to make large sales volume on their behalf instead of use of direct selling. This
channel strategy includes system retailers which helps the top-level managers to propel their
partner’s sales processes and lower the threat of having company duplicates (Fotopoulos &
Psomas 2009). This tool should be successfully implemented to create harmonious collaboration
of manufacturers, vendors and customers thus creating a profitable partnership
6.1.4. The business model canvas
This involves development of new modification of the available company models. The
best partner company for the manufacture of probiotics will be provide the visual chart that
consist of elements of the company product value, customers, transportation means and financial
resources. (Fotopoulos & Psomas, 2009). This model will also involve how a company will
generate revenue streams such as asset sale, leasing and advertising through media. The business
Model canvas ought to be printed on the company board in order to identify the best partner
(Rigby & Bilodeau, 2011).
6.1.5. Partnerships
Partnership relationship management tool involves the existing processes and strategies
that a company utilize in directing the operations with partners who make product sales. This
approach includes partner portal, consumer computer databases that ensure various companies
and partners in management of sales metrics, prices and inventories of the manufacture product
(Fotopoulos & Psomas, 2009). Probiotic manufacturing companies should trust on partner
companies to make large sales volume on their behalf instead of use of direct selling. This
channel strategy includes system retailers which helps the top-level managers to propel their
partner’s sales processes and lower the threat of having company duplicates (Fotopoulos &
Psomas 2009). This tool should be successfully implemented to create harmonious collaboration
of manufacturers, vendors and customers thus creating a profitable partnership
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING10
7. The Methodology of the Work
7.1. The manufacturing of the probiotics
The research considered different criteria used during the manufacture of probiotics in
different companies. Some of the companies considered were: The Bio-Cat microbial, The
NutraScience Labs, The Terragens Research and the Multikraft probiotics in Australia.
Different criteria were used to measure the percentage of production of the probiotics in
the companies mentioned above. The criteria used was to identify how the following measures
have been employed by each company so as to come up with the suitable company for the
production of the probiotics (Sanders, 2008).
7.2. Selection criteria for each company
In order to obtain a good research some of the criteria considered in selecting the suitable
company for manufacturing of the probiotics were:
Checking whether the company participates in the external contracts or the toll
manufacturing processes.
Screening the scale assigned to each equipment used during the fermentation process.
Down streaming the processing equipment used in each company that is the use of the
centrifuges and the dryers used.
Locating the experience and the cost incurred. Also, the aspect of the experience on
dealing with the probiotics was analyzed within the company selected during the
research.
Determining the issue of management and the quality system outlined by each company.
That is measuring the standards used by the companies in manufacturing of the
probiotics.
7. The Methodology of the Work
7.1. The manufacturing of the probiotics
The research considered different criteria used during the manufacture of probiotics in
different companies. Some of the companies considered were: The Bio-Cat microbial, The
NutraScience Labs, The Terragens Research and the Multikraft probiotics in Australia.
Different criteria were used to measure the percentage of production of the probiotics in
the companies mentioned above. The criteria used was to identify how the following measures
have been employed by each company so as to come up with the suitable company for the
production of the probiotics (Sanders, 2008).
7.2. Selection criteria for each company
In order to obtain a good research some of the criteria considered in selecting the suitable
company for manufacturing of the probiotics were:
Checking whether the company participates in the external contracts or the toll
manufacturing processes.
Screening the scale assigned to each equipment used during the fermentation process.
Down streaming the processing equipment used in each company that is the use of the
centrifuges and the dryers used.
Locating the experience and the cost incurred. Also, the aspect of the experience on
dealing with the probiotics was analyzed within the company selected during the
research.
Determining the issue of management and the quality system outlined by each company.
That is measuring the standards used by the companies in manufacturing of the
probiotics.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING11
8. Results and discussion
Most of the companies deal with the large production of the probiotics. The whole
process of manufacturing the probiotics is characterized by the use of advanced technology that
is employed in the fermentation process and use of efficient machines for drying. Some of the
companies have a large number of the laboratory that helps to do research on the probiotics.
8.1. Fermentation process used
Fermentation procedures are used to in either manufacture the microbial products in
either large quantity or even in small quantity. The commonly used microbial by the companies
mentioned are the enzymes, the amino acids, use of the vitamins, and also use the relevant
pharmaceutical products. Different studies reveal that this company uses the research from the
labs to design the probiotics. Here the concept of the quality assurance runs all through to each
laboratory within the company.
8.2. Fermentation methods used by the companies
Fermentation can be done in two different ways. It can either be through the continuous
fermentation or the batch fermentation.In the batch fermentation, all the components used are
first sterilized and there is mixing of the inoculum in the fermenter and then is kept in optimum
temperature to allow the growth of the probiotic. This is common in companies such as The Bio-
Cat microbial and The NutraScience Labs. During the fermentation different nutrients are added
so as to facilitate the growth of the micro-organisms used. A base medium is used also to
establish PH medium needed for fermentation, therefore, helping the function of the
microorganism within the fermentation chamber (Turgut & Cakmakci, 2009). After the
completion of the fermentation, process cells are recovered from the centrifugal chambers
through the filtration method. The main objective in batch fermentation is the issue of coming up
8. Results and discussion
Most of the companies deal with the large production of the probiotics. The whole
process of manufacturing the probiotics is characterized by the use of advanced technology that
is employed in the fermentation process and use of efficient machines for drying. Some of the
companies have a large number of the laboratory that helps to do research on the probiotics.
8.1. Fermentation process used
Fermentation procedures are used to in either manufacture the microbial products in
either large quantity or even in small quantity. The commonly used microbial by the companies
mentioned are the enzymes, the amino acids, use of the vitamins, and also use the relevant
pharmaceutical products. Different studies reveal that this company uses the research from the
labs to design the probiotics. Here the concept of the quality assurance runs all through to each
laboratory within the company.
8.2. Fermentation methods used by the companies
Fermentation can be done in two different ways. It can either be through the continuous
fermentation or the batch fermentation.In the batch fermentation, all the components used are
first sterilized and there is mixing of the inoculum in the fermenter and then is kept in optimum
temperature to allow the growth of the probiotic. This is common in companies such as The Bio-
Cat microbial and The NutraScience Labs. During the fermentation different nutrients are added
so as to facilitate the growth of the micro-organisms used. A base medium is used also to
establish PH medium needed for fermentation, therefore, helping the function of the
microorganism within the fermentation chamber (Turgut & Cakmakci, 2009). After the
completion of the fermentation, process cells are recovered from the centrifugal chambers
through the filtration method. The main objective in batch fermentation is the issue of coming up

PARTNERSHIPS FOR PROBIOTICS MANUFACTURING12
with the cellular concentration that will give a high percentage of the probiotics. In most cases,
the concentration may tend to have a high viscosity which is compensated by adding the
vegetative materials so as to recover the cells and maintaining the appropriate pH thus increased
production of the probiotics (Kudełka, 2010).
The Terragens Research and the Multikraft probiotics in Australia use the continuous
fermentation whereby the fresh growing materials are incorporated with the culture and the cells
of the bacteria and even other substrates which will be occasionally removed so as to facilitate
the production of the probiotics. The change of genetic composure is facilitated through the
mutation process. This is always through the contamination of the bacteria and the substrate
contained in the fermentation chamber.
8.3. Drying
This is commonly used after the fermentation process. The yeast and the bacteria cells are
dried thus enhancing the transportation process. The probiotics are dried through the use of the
freeze or the spray drying mechanism. The issue of keeping the viability of cells is crucial when
drying so as to ensure that there is better probiotic production within the company (Kudełka,
2010).
In freeze-drying, there is the use of the nitrogen or the dry ice and even use of
refrigerators at a temperature of 200 °C. The process is performed at a high temperature as to
prevent the formation of the ice within the chambers. In spray drying, most of the companies use
a heated nozzle which is sprayed to the drying chamber against the hot air passed through the
chambers. The technique is commonly used since it has low cost as compared to the freeze-
drying method.
with the cellular concentration that will give a high percentage of the probiotics. In most cases,
the concentration may tend to have a high viscosity which is compensated by adding the
vegetative materials so as to recover the cells and maintaining the appropriate pH thus increased
production of the probiotics (Kudełka, 2010).
The Terragens Research and the Multikraft probiotics in Australia use the continuous
fermentation whereby the fresh growing materials are incorporated with the culture and the cells
of the bacteria and even other substrates which will be occasionally removed so as to facilitate
the production of the probiotics. The change of genetic composure is facilitated through the
mutation process. This is always through the contamination of the bacteria and the substrate
contained in the fermentation chamber.
8.3. Drying
This is commonly used after the fermentation process. The yeast and the bacteria cells are
dried thus enhancing the transportation process. The probiotics are dried through the use of the
freeze or the spray drying mechanism. The issue of keeping the viability of cells is crucial when
drying so as to ensure that there is better probiotic production within the company (Kudełka,
2010).
In freeze-drying, there is the use of the nitrogen or the dry ice and even use of
refrigerators at a temperature of 200 °C. The process is performed at a high temperature as to
prevent the formation of the ice within the chambers. In spray drying, most of the companies use
a heated nozzle which is sprayed to the drying chamber against the hot air passed through the
chambers. The technique is commonly used since it has low cost as compared to the freeze-
drying method.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 44
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





