Mass Spectrometry: Principles, Applications in Forensic Science
VerifiedAdded on 2023/06/05
|2
|303
|337
Report
AI Summary
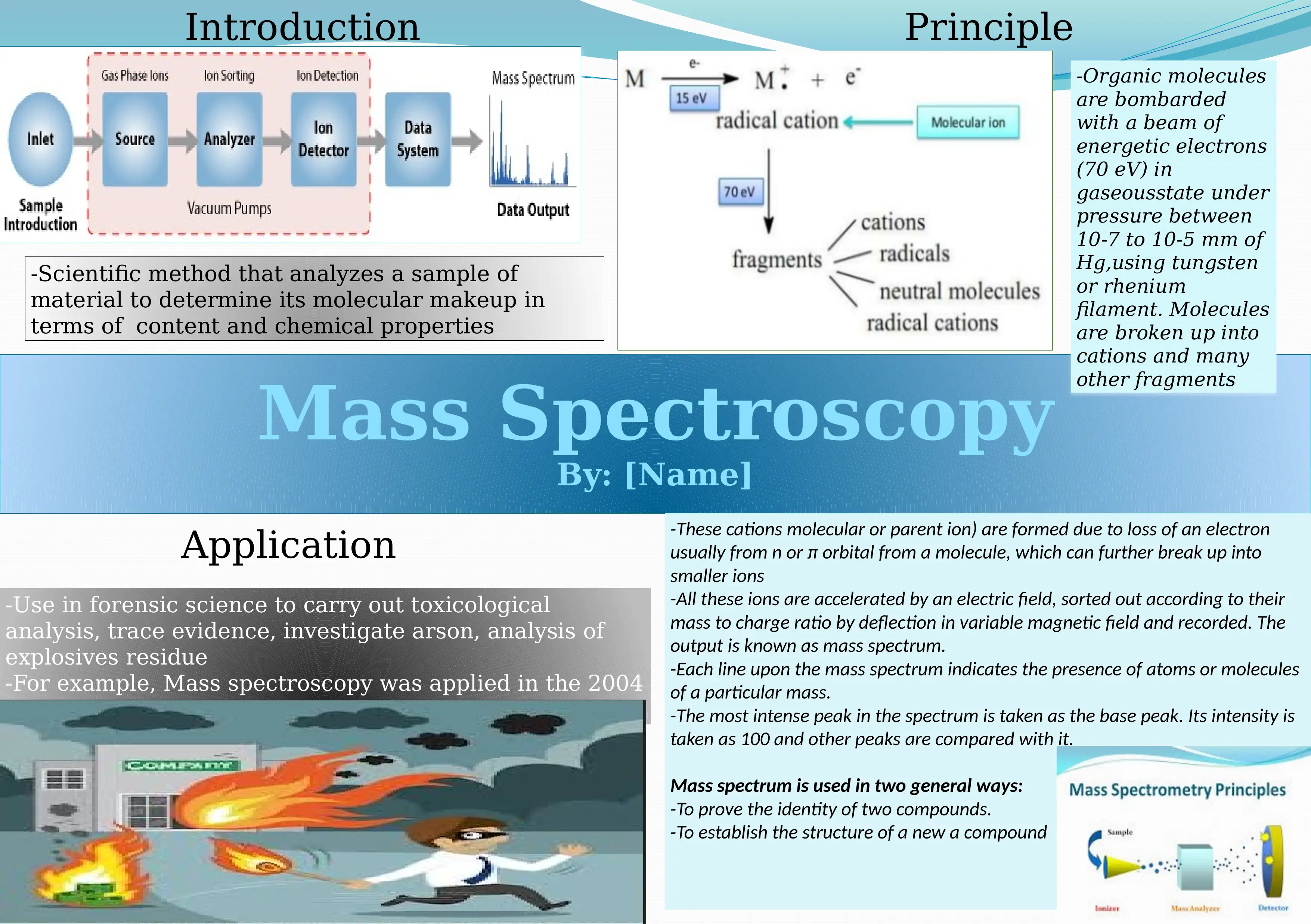
This report provides an overview of mass spectrometry and its applications in forensic science. Mass spectrometry is a scientific method used to analyze a sample of material to determine its molecular makeup in terms of content and chemical properties. The process involves bombarding organic molecules with energetic electrons, breaking them into cations and fragments, which are then accelerated through an electric field and sorted by their mass-to-charge ratio. The resulting mass spectrum identifies the presence of specific atoms or molecules. Applications include toxicological analysis, trace evidence investigation, arson analysis, and explosives residue analysis, as demonstrated in the 2004 Cameron Todd Willingham arson case. The report also details how to interpret a mass spectrum, including the identification of the base peak and molecular ions. Desklib offers a platform for students to access similar solved assignments and study resources.
1 out of 2




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)