Laboratory Report: Experiment 1 - Mass, Volume, Density, and Pipette
VerifiedAdded on 2023/06/07
|6
|1162
|293
AI Summary
This laboratory report details an experiment focused on calibrating a Pasteur pipette and determining the mass, volume, and density of various substances. The experiment involved calibrating Pasteur pipettes, measuring the mass of sodium chloride using different weighing techniques, calculating the density of distilled water using various glassware, and estimating the density of an unknown organic liquid. Observations included differences in drop rates between broken and unbroken pipettes and the effectiveness of folded versus unfolded weighing papers. The results section presents data on droplet counts, mass measurements, and density calculations. The discussion emphasizes the importance of proper techniques and observation skills in achieving accurate results. The experiment successfully demonstrated the principles of density calculation and substance identification, despite potential sources of error. Desklib offers a wealth of similar lab reports and solved assignments to aid students in their studies.

Name:
Institution:
Tutor:
Date:
Laboratory Report
Experiment1: Mass, Volume, Density and Pasteur Pipette Calibration
Aims: the aim of the experiment was to calibrate a Pasteur pipette correctly so as to estimate the
mass and volume of a substance and eventually calculate its density.
Procedure
Part 1) calibration of the Pasteur pipette
1. Distilled water was added to 10 ml cylinder using a clean Pasteur pipette drop by drop
while controlling to avoid any excess on the walls of the cylinders to reduce water
remaining on walls of the cylinder. The amount of droplets was counted and the
observations recorded.
2. The cylinder was cleaned properly and the liquid disposed properly. The cylinder was dry
cleaned and the process was repeated again.
3. Step 1 was repeated but this time with a new pipette. The amount of droplets needed to
fill the cylinder were noted and compared with the first trial.
4. The tips of the pipettes were broken abut not at the same spot.
5. Step 1 was repeated using broken pipettes and the amount of droplets needed to fill the
cylinder were noted.
Institution:
Tutor:
Date:
Laboratory Report
Experiment1: Mass, Volume, Density and Pasteur Pipette Calibration
Aims: the aim of the experiment was to calibrate a Pasteur pipette correctly so as to estimate the
mass and volume of a substance and eventually calculate its density.
Procedure
Part 1) calibration of the Pasteur pipette
1. Distilled water was added to 10 ml cylinder using a clean Pasteur pipette drop by drop
while controlling to avoid any excess on the walls of the cylinders to reduce water
remaining on walls of the cylinder. The amount of droplets was counted and the
observations recorded.
2. The cylinder was cleaned properly and the liquid disposed properly. The cylinder was dry
cleaned and the process was repeated again.
3. Step 1 was repeated but this time with a new pipette. The amount of droplets needed to
fill the cylinder were noted and compared with the first trial.
4. The tips of the pipettes were broken abut not at the same spot.
5. Step 1 was repeated using broken pipettes and the amount of droplets needed to fill the
cylinder were noted.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Part 2) Measuring of mass
1. Checked if the balance was clean
2. A weighing paper that had not been folded was placed on the weighing balance
3. The balance was carefully tared
4. 1.00g of sodium chloride was measured.
5. The solid together with the paper were carefully transferred to the beaker avoiding any
spills.
6. Step 4 was repeated but this time the weighing paper was folded. A comparison was
made to find out the most effective.
Part 3) Measurement of volume: calculation of Density.
1. A new 50ml beaker was placed on a clean balance then tared
2. The beaker was removed and the balance reset.
3. 10 ml distilled water was then added to the beaker
4. The mass of beaker and that of distilled water was then recorded.
5. The data obtained was the one to be used to calculate the density.
6. Steps 1-5 were then repeated but this time without a pipette.
7. The water was disposed in the sink and the glassware dried then prepared for the
subsequent steps.
8. 10 ml graduated pipette was used in repeating procedure of part one of the experiment
9. The density of the water was then compared in the previous glassware
Part 4) Estimating the density of organic liquid.
1. Checked if the balance was clean
2. A weighing paper that had not been folded was placed on the weighing balance
3. The balance was carefully tared
4. 1.00g of sodium chloride was measured.
5. The solid together with the paper were carefully transferred to the beaker avoiding any
spills.
6. Step 4 was repeated but this time the weighing paper was folded. A comparison was
made to find out the most effective.
Part 3) Measurement of volume: calculation of Density.
1. A new 50ml beaker was placed on a clean balance then tared
2. The beaker was removed and the balance reset.
3. 10 ml distilled water was then added to the beaker
4. The mass of beaker and that of distilled water was then recorded.
5. The data obtained was the one to be used to calculate the density.
6. Steps 1-5 were then repeated but this time without a pipette.
7. The water was disposed in the sink and the glassware dried then prepared for the
subsequent steps.
8. 10 ml graduated pipette was used in repeating procedure of part one of the experiment
9. The density of the water was then compared in the previous glassware
Part 4) Estimating the density of organic liquid.

1. An empty capped 3ml vial was placed on a pan and the mass was recorded.0.50 ml of the
organic liquid was then drawn up.
2. The 0.500ml of the organic liquid was then released into a small amount back into the
reservoir .Made sure that the reservoir read 0.500ml and the liquid was then transferred to
the vial and the weight recorded.
3. The density was then calculated and cleaned the spilled liquid
4. Table 1B-1 was then used to determine the unknown liquid.
Observations
To find out if there is a difference in the drop rate, two Pasteur pipettes were used and then the
tip of one of them broken. Results showed that the pipette with a broken tip is faster in dropping
the liquid than the one which is not broken. Another unique observation was that whenever a
liquid is being transferred to another container, 0.1ml of the liquid would get lost and this can
lead to errors in the final results. To determine the best method to transfer a solid mass to a
container, unfolded and folded papers were used. The results indicated that using a folded paper
is much easier.
RESULTS
Part 1:
Pipette 1 Pipette 2
Original Pipette(Number of
drops to 1ml)
33 30
Broken Tip(Number of drops
to 1mL)
33 30
organic liquid was then drawn up.
2. The 0.500ml of the organic liquid was then released into a small amount back into the
reservoir .Made sure that the reservoir read 0.500ml and the liquid was then transferred to
the vial and the weight recorded.
3. The density was then calculated and cleaned the spilled liquid
4. Table 1B-1 was then used to determine the unknown liquid.
Observations
To find out if there is a difference in the drop rate, two Pasteur pipettes were used and then the
tip of one of them broken. Results showed that the pipette with a broken tip is faster in dropping
the liquid than the one which is not broken. Another unique observation was that whenever a
liquid is being transferred to another container, 0.1ml of the liquid would get lost and this can
lead to errors in the final results. To determine the best method to transfer a solid mass to a
container, unfolded and folded papers were used. The results indicated that using a folded paper
is much easier.
RESULTS
Part 1:
Pipette 1 Pipette 2
Original Pipette(Number of
drops to 1ml)
33 30
Broken Tip(Number of drops
to 1mL)
33 30
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
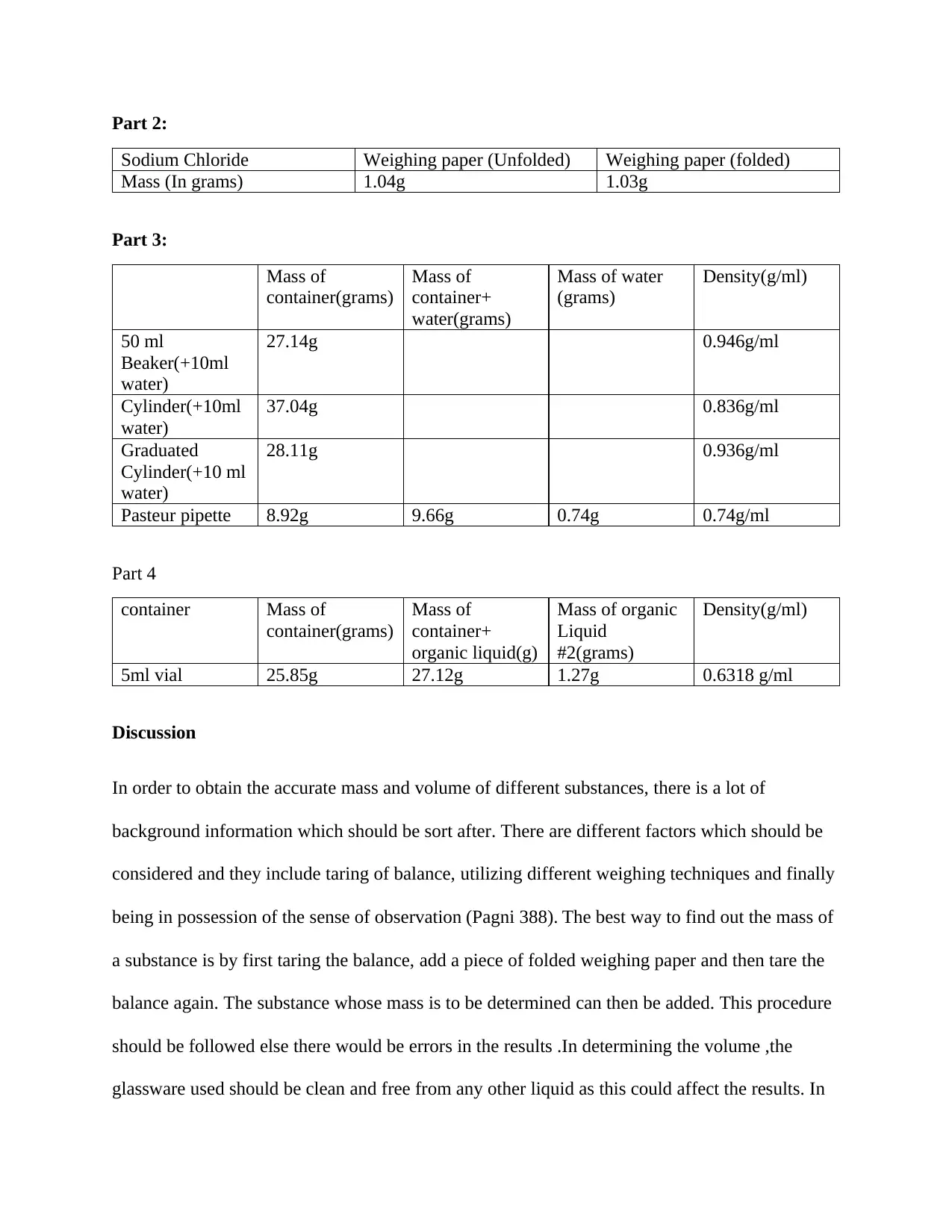
Part 2:
Sodium Chloride Weighing paper (Unfolded) Weighing paper (folded)
Mass (In grams) 1.04g 1.03g
Part 3:
Mass of
container(grams)
Mass of
container+
water(grams)
Mass of water
(grams)
Density(g/ml)
50 ml
Beaker(+10ml
water)
27.14g 0.946g/ml
Cylinder(+10ml
water)
37.04g 0.836g/ml
Graduated
Cylinder(+10 ml
water)
28.11g 0.936g/ml
Pasteur pipette 8.92g 9.66g 0.74g 0.74g/ml
Part 4
container Mass of
container(grams)
Mass of
container+
organic liquid(g)
Mass of organic
Liquid
#2(grams)
Density(g/ml)
5ml vial 25.85g 27.12g 1.27g 0.6318 g/ml
Discussion
In order to obtain the accurate mass and volume of different substances, there is a lot of
background information which should be sort after. There are different factors which should be
considered and they include taring of balance, utilizing different weighing techniques and finally
being in possession of the sense of observation (Pagni 388). The best way to find out the mass of
a substance is by first taring the balance, add a piece of folded weighing paper and then tare the
balance again. The substance whose mass is to be determined can then be added. This procedure
should be followed else there would be errors in the results .In determining the volume ,the
glassware used should be clean and free from any other liquid as this could affect the results. In
Sodium Chloride Weighing paper (Unfolded) Weighing paper (folded)
Mass (In grams) 1.04g 1.03g
Part 3:
Mass of
container(grams)
Mass of
container+
water(grams)
Mass of water
(grams)
Density(g/ml)
50 ml
Beaker(+10ml
water)
27.14g 0.946g/ml
Cylinder(+10ml
water)
37.04g 0.836g/ml
Graduated
Cylinder(+10 ml
water)
28.11g 0.936g/ml
Pasteur pipette 8.92g 9.66g 0.74g 0.74g/ml
Part 4
container Mass of
container(grams)
Mass of
container+
organic liquid(g)
Mass of organic
Liquid
#2(grams)
Density(g/ml)
5ml vial 25.85g 27.12g 1.27g 0.6318 g/ml
Discussion
In order to obtain the accurate mass and volume of different substances, there is a lot of
background information which should be sort after. There are different factors which should be
considered and they include taring of balance, utilizing different weighing techniques and finally
being in possession of the sense of observation (Pagni 388). The best way to find out the mass of
a substance is by first taring the balance, add a piece of folded weighing paper and then tare the
balance again. The substance whose mass is to be determined can then be added. This procedure
should be followed else there would be errors in the results .In determining the volume ,the
glassware used should be clean and free from any other liquid as this could affect the results. In
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

the first part of the lab, it was found out that using a pipette with a broken tip would need few
droplets to fill 1ml of the substance. In part 2 of the experiment, it was established that using a
folded paper is more accurate than the unfolded paper due to increased stability.
Conclusion
The lesson learnt in the experiment was that it is actually possible to calculate the density of
different substances by measuring both the weight as well as the volume of the particular
substances .In the experiment, there are likely to be sources of error and therefore the values
obtained are not accurate but they are almost similar to those expected. Despite the errors
however, the objective of the experiment were achieved since the density for each substance was
calculated. The unknown organic liquid was also identified.
droplets to fill 1ml of the substance. In part 2 of the experiment, it was established that using a
folded paper is more accurate than the unfolded paper due to increased stability.
Conclusion
The lesson learnt in the experiment was that it is actually possible to calculate the density of
different substances by measuring both the weight as well as the volume of the particular
substances .In the experiment, there are likely to be sources of error and therefore the values
obtained are not accurate but they are almost similar to those expected. Despite the errors
however, the objective of the experiment were achieved since the density for each substance was
calculated. The unknown organic liquid was also identified.

Works Cited
Pagni, Richard M. "Techniques in Organic Chemistry: Miniscale, Standard Taper
Microscale, and Williamson Microscale (Mohrig, Jerry R.; Hammond, Christina
Noring; Schatz, Paul F.; Morrill, Terence C.)." Journal of Chemical Education,
vol. 80, no. 4, 2003, p. 388.
Pagni, Richard M. "Techniques in Organic Chemistry: Miniscale, Standard Taper
Microscale, and Williamson Microscale (Mohrig, Jerry R.; Hammond, Christina
Noring; Schatz, Paul F.; Morrill, Terence C.)." Journal of Chemical Education,
vol. 80, no. 4, 2003, p. 388.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





