Thermodynamics, Combustion, and Compressor Analysis Homework Solution
VerifiedAdded on 2020/04/21
|7
|481
|108
Homework Assignment
AI Summary
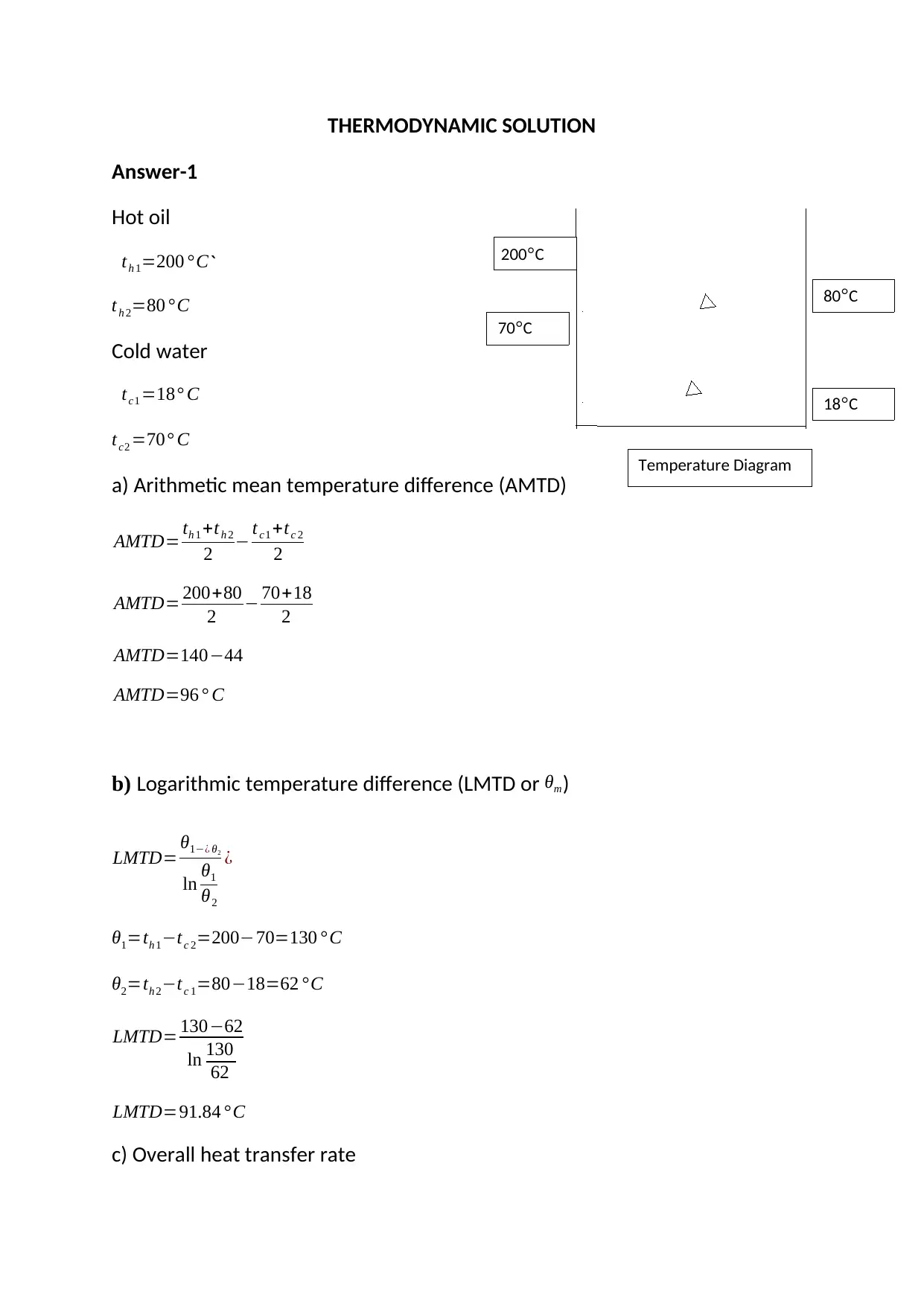
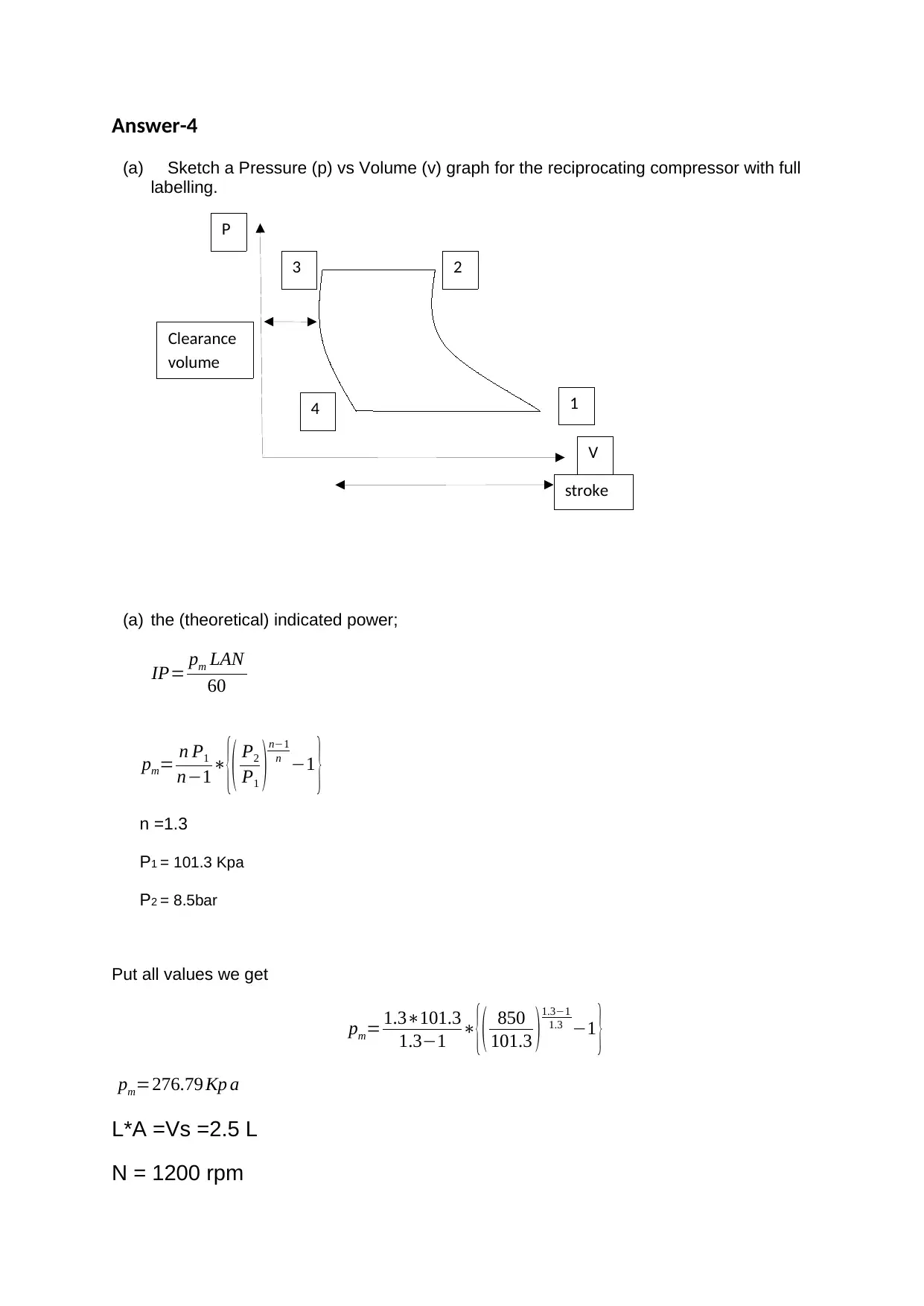
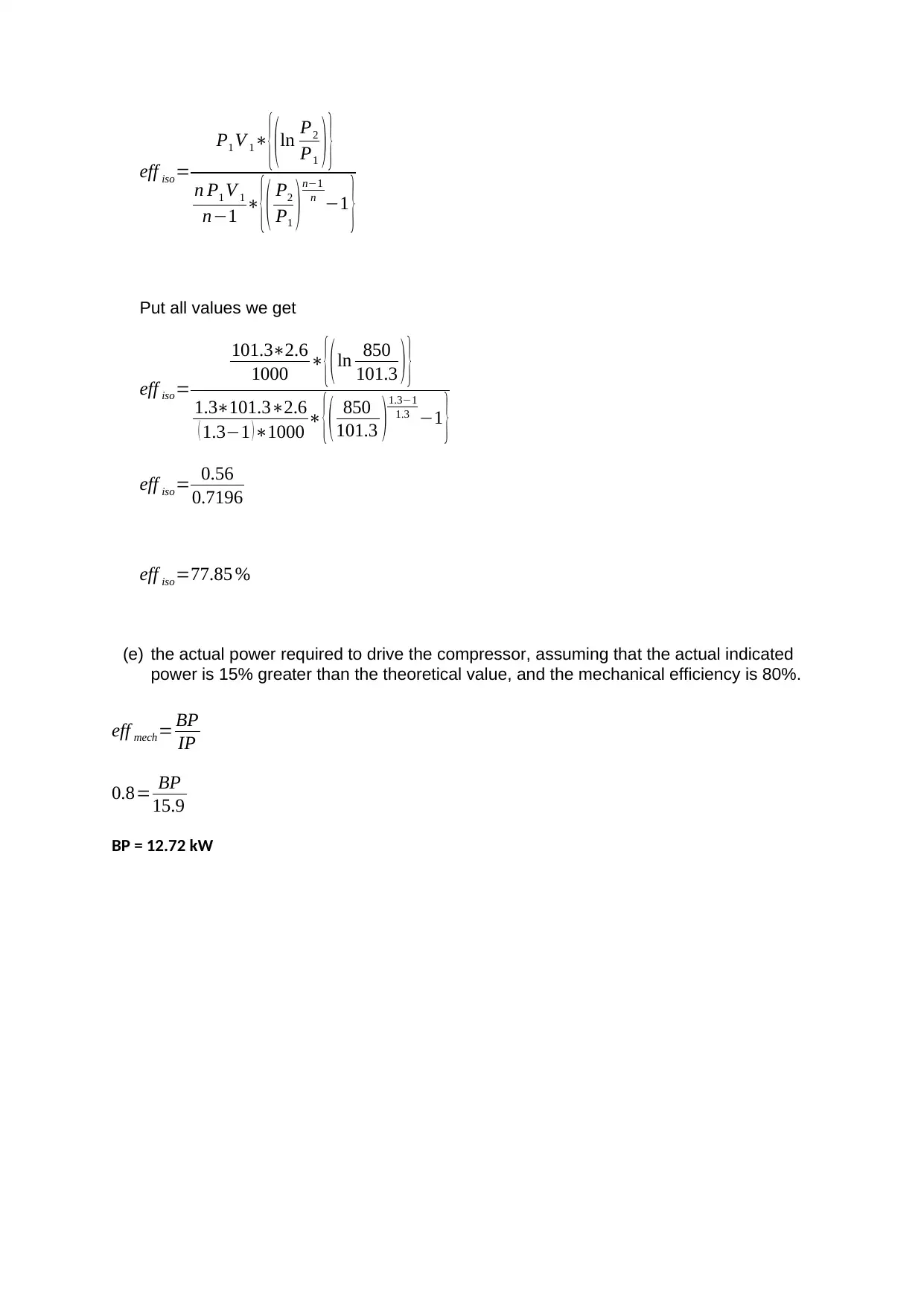
This document presents solutions to a mechanical engineering homework assignment. The first part addresses heat transfer calculations involving hot oil and cold water, determining the arithmetic mean temperature difference (AMTD), logarithmic temperature difference (LMTD), overall heat transfer rate, and mass flow rates. The second part focuses on a mass balance for the stoichiometric combustion of ethanol, calculating the mass of oxygen required, the mass of air and nitrogen, the air/fuel ratio, and the mass of carbon dioxide and water produced. The third part involves calculating the mass flow rate of ethanol. The final part analyzes a reciprocating compressor, including a pressure-volume graph, calculations for indicated power, volumetric efficiency, mass flow rate, isothermal efficiency, and the actual power required considering mechanical efficiency.
1 out of 7





![[object Object]](/_next/static/media/star-bottom.7253800d.svg)