Mechanical Engineering Thermodynamics Quiz Solution - University Name
VerifiedAdded on 2022/07/28
|10
|282
|23
Quiz and Exam
AI Summary
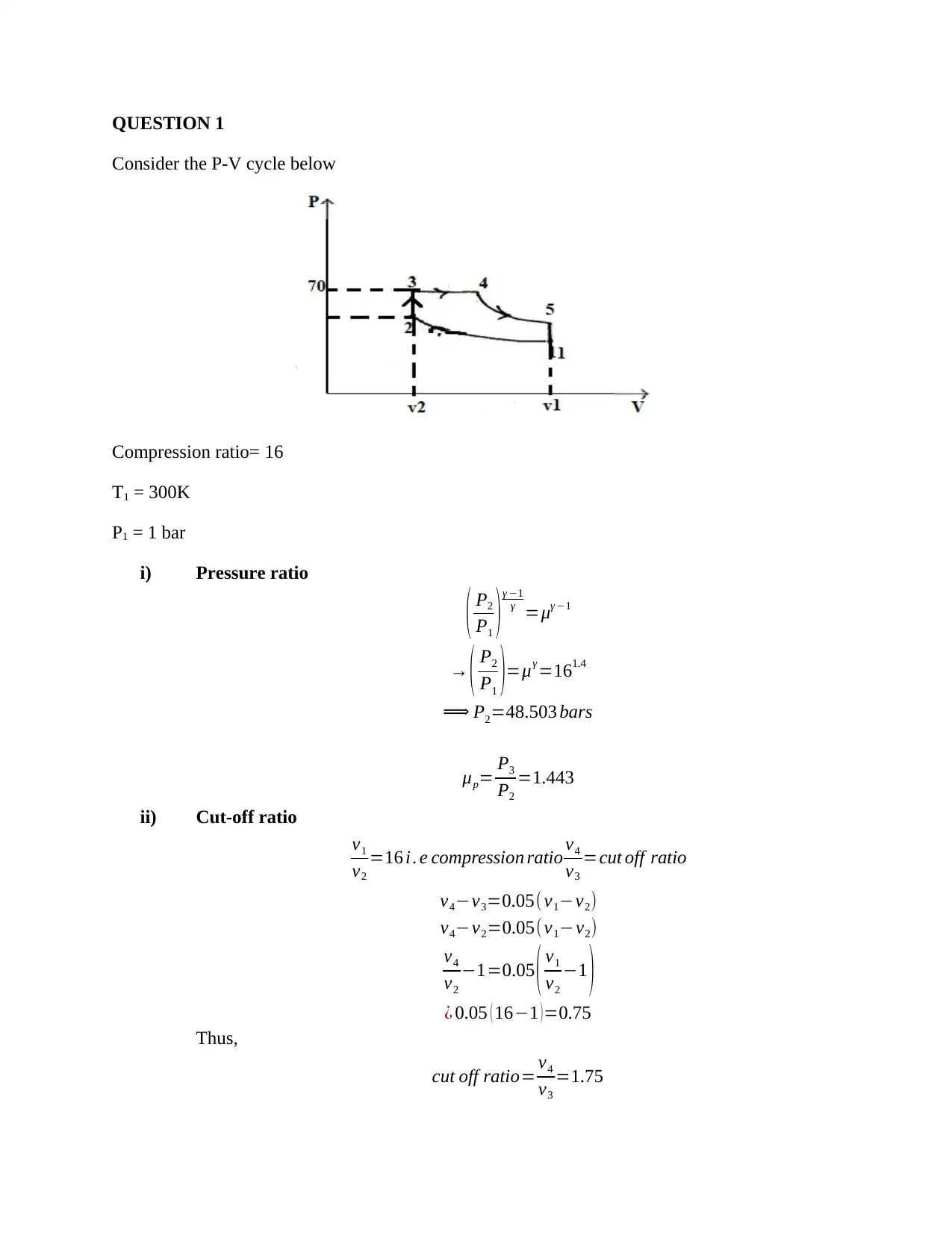
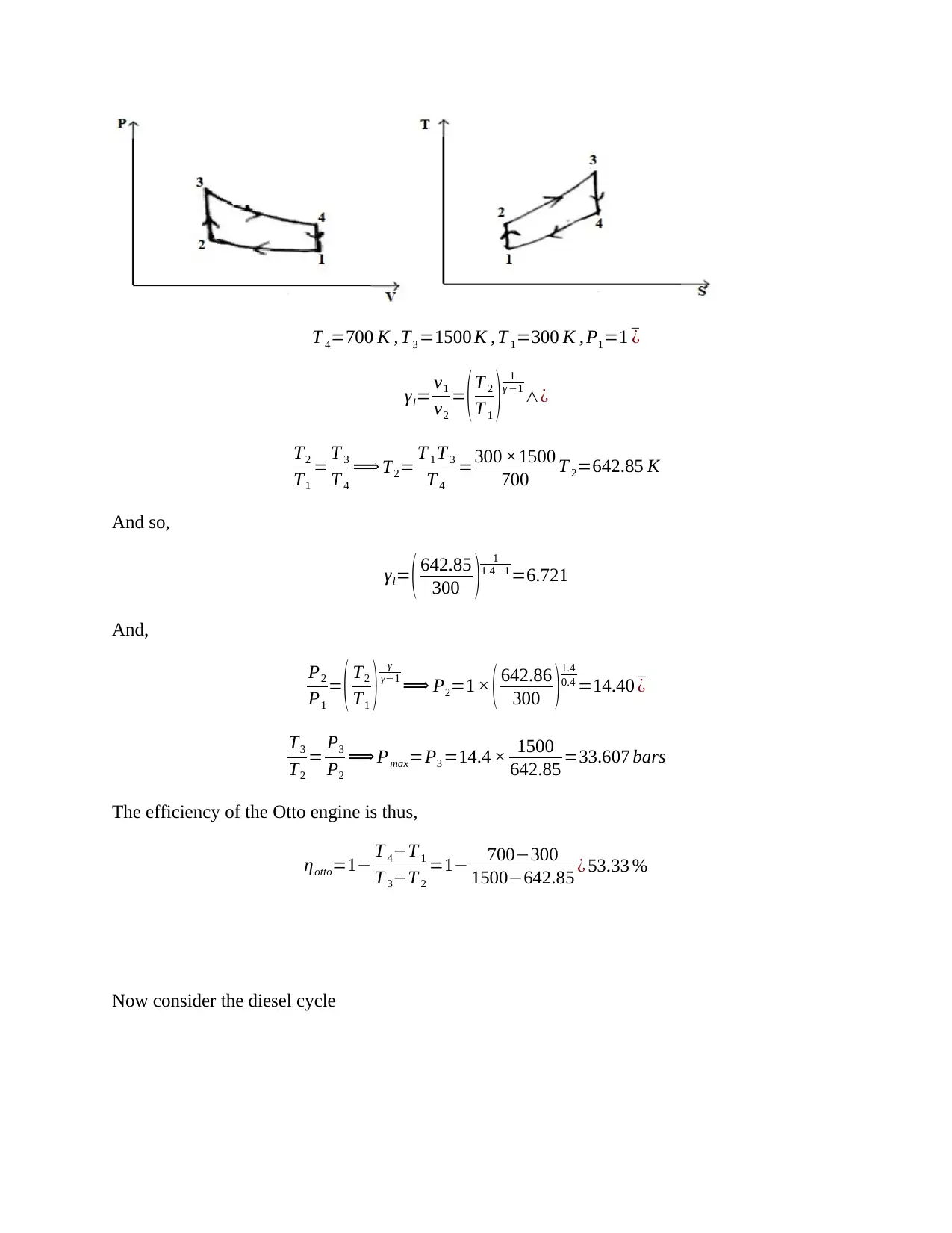
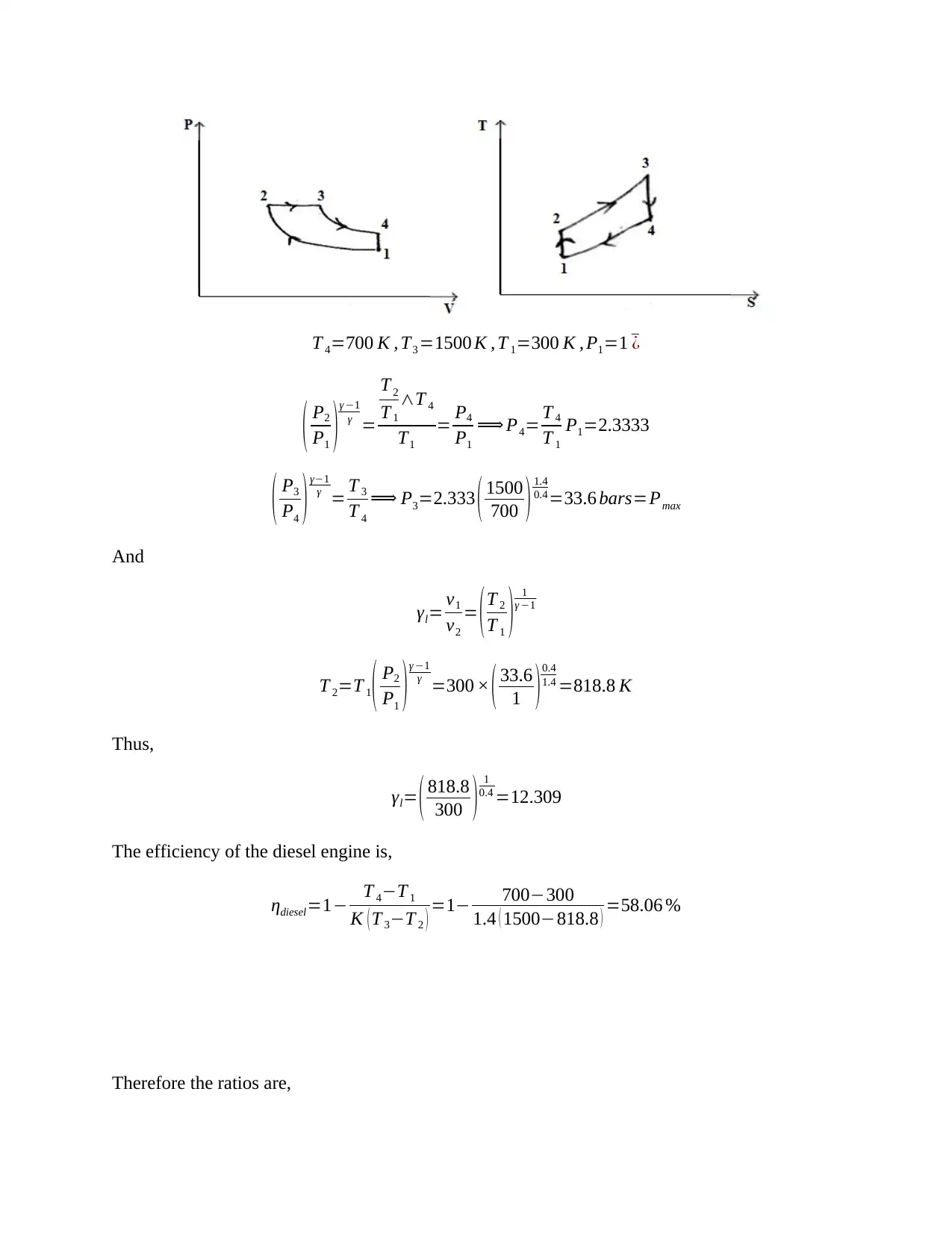
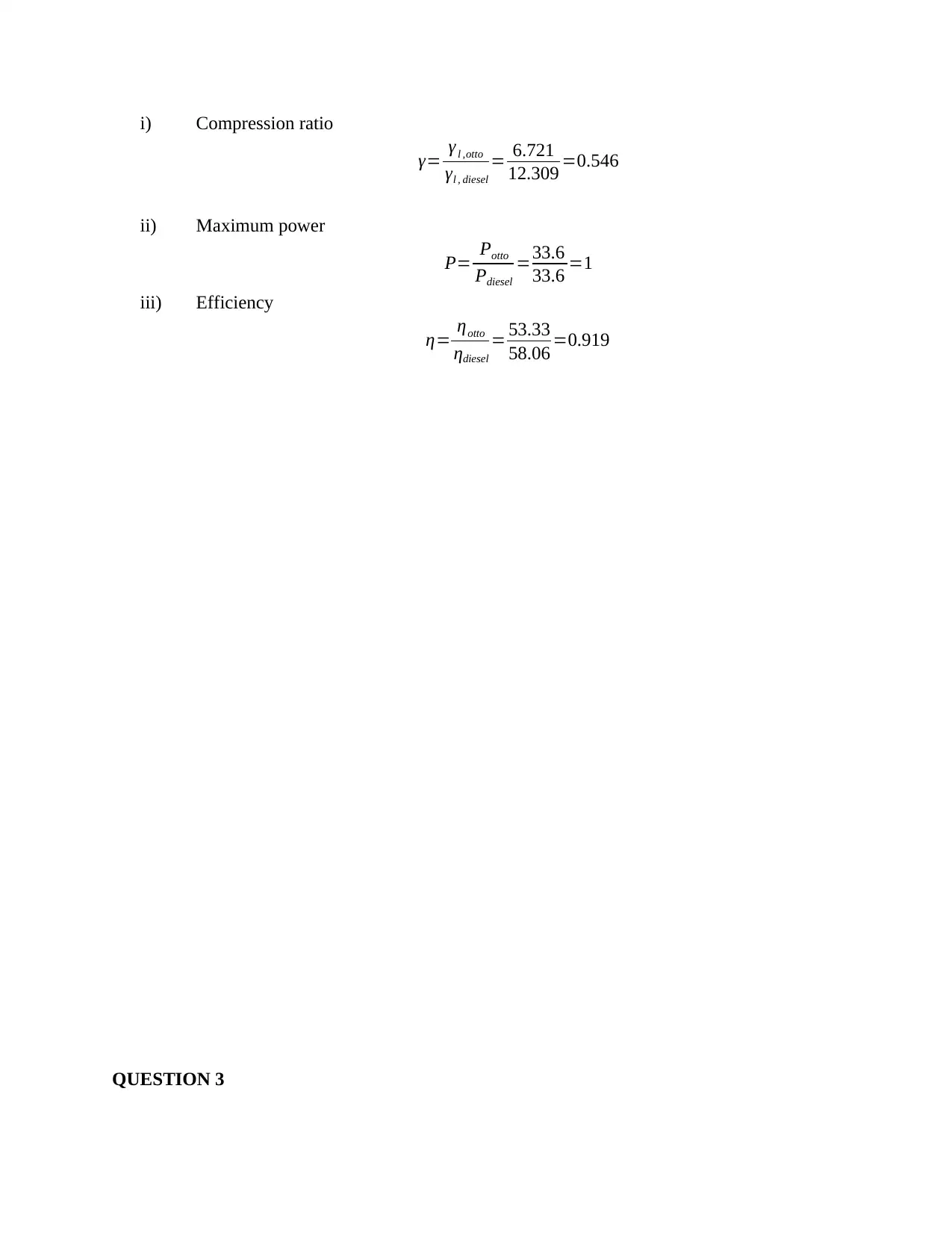
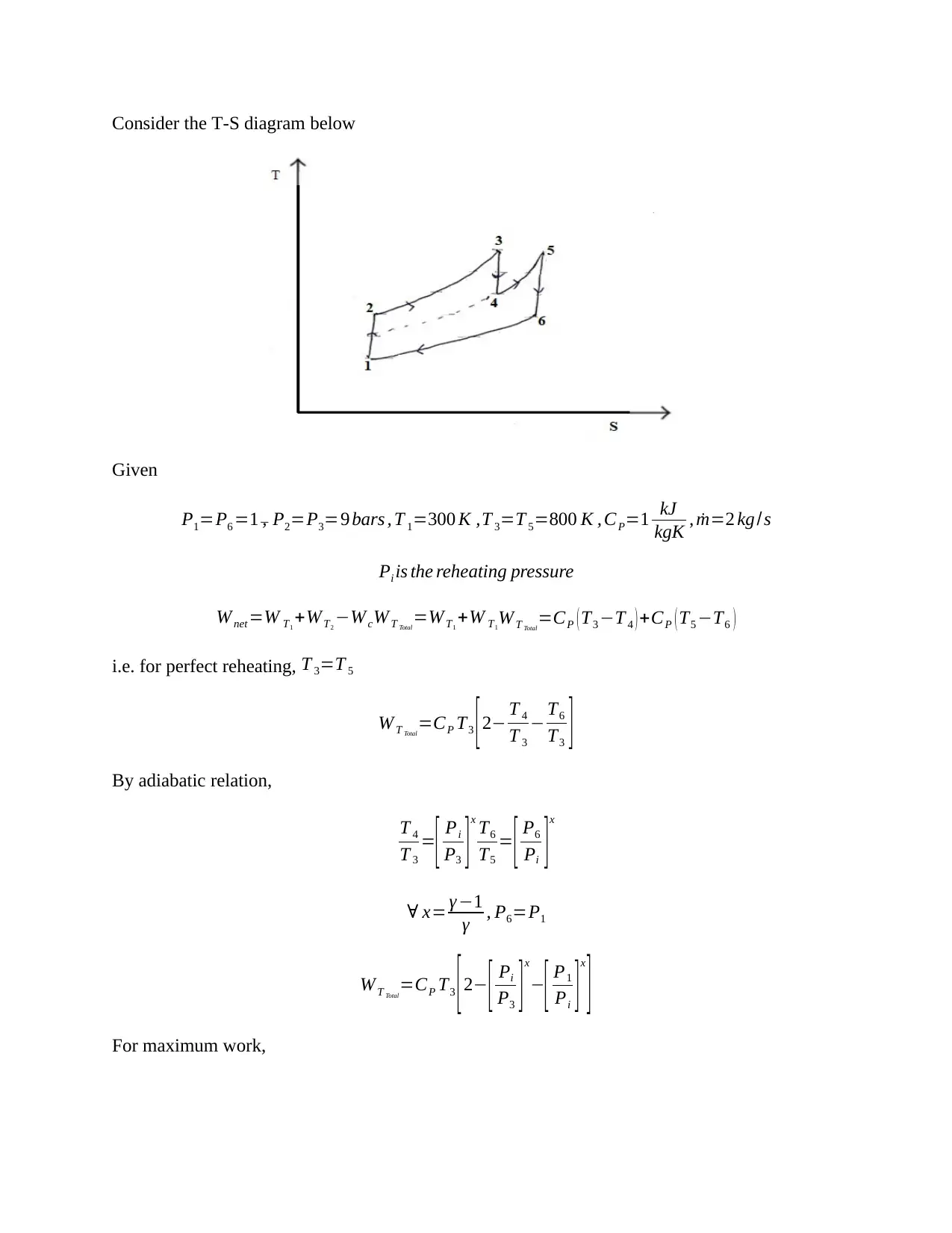
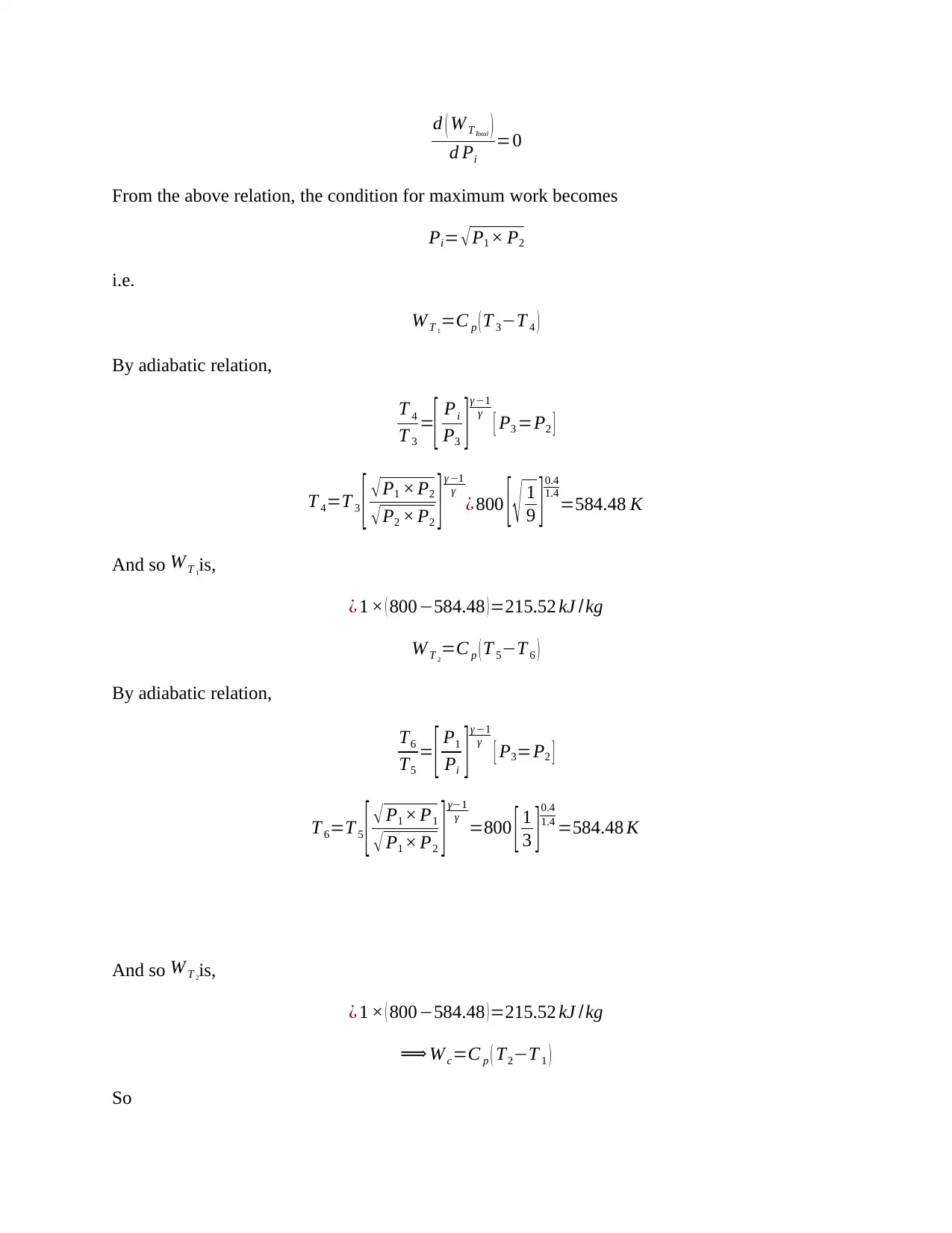
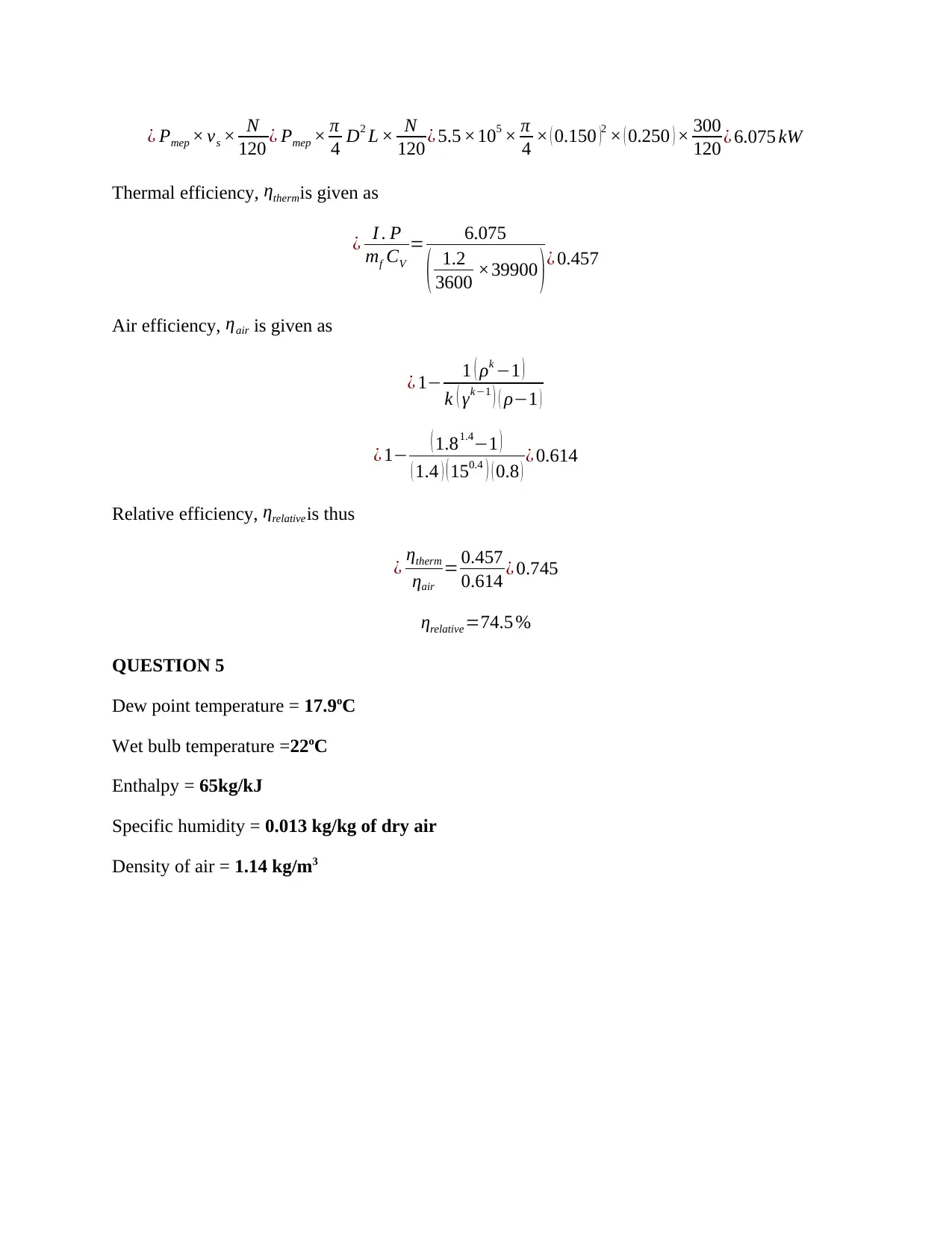
This document presents a detailed solution to a Thermodynamics quiz, addressing key concepts in the field. The solution covers calculations for an ideal dual cycle engine, including pressure and cut-off ratios, heat transfer, work done, and thermal efficiency. It also includes an analysis of Otto and Diesel cycles, determining the ratios of compression ratio, maximum pressure, and efficiency. Furthermore, the solution delves into gas turbine plant analysis, calculating adiabatic relations, and maximum work conditions. Finally, the document includes calculations for engine performance parameters, such as indicated power, thermal efficiency, and air efficiency, for a given engine configuration. This resource is invaluable for students seeking to understand and master the principles of thermodynamics and engine performance.
1 out of 10




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)