Medical Devices Company: Logistics and Distribution Strategies
VerifiedAdded on 2022/10/10
|16
|3101
|28
Report
AI Summary
This report provides an extensive analysis of a medical devices company operating in the European market, addressing current market trends and challenges. It delves into the company's overview, including its manufacturing processes, distribution network, and the issues it faces, such as inventory turnover and product obsolescence. The core of the report focuses on exploring effective logistics management strategies, including segmented supply chains, direct distribution models, shared services, and outsourcing models using 3PL providers. The report highlights the benefits of these strategies, such as cost efficiency, network flexibility, and improved supply chain visibility. It also emphasizes the importance of personnel training, change control, and internal audits to ensure the successful implementation of these strategies. The report concludes by underscoring the importance of adapting to the evolving market to maintain and improve the company's success.

Running Head: THE MEDICAL DEVICES COMPANY
The medical devices company
Name of the University:
Name of the Student:
Authors Note:
The medical devices company
Name of the University:
Name of the Student:
Authors Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1THE MEDICAL DEVICES COMPANY
Executive Summary
This article deals with the Extensive study of the Medical Devices Company of the European
market. The current trends of the market. It also highlights the logistics management
strategies that can be used to optimize the distribution and transportation of the medical
devices company ensuring their success.
Executive Summary
This article deals with the Extensive study of the Medical Devices Company of the European
market. The current trends of the market. It also highlights the logistics management
strategies that can be used to optimize the distribution and transportation of the medical
devices company ensuring their success.

2THE MEDICAL DEVICES COMPANY
Table of Contents
Introduction................................................................................................................................3
Media Devices Company: An Overview and related issues......................................................4
Logistic management strategy....................................................................................................5
The segmented supply chain..................................................................................................5
The direct distribution model.................................................................................................6
Adding shared services..........................................................................................................8
The outsourcing model...........................................................................................................9
Benefits of 3PL....................................................................................................................10
Personnel and staff training..................................................................................................10
Key personnel...................................................................................................................10
Training............................................................................................................................11
Change control.....................................................................................................................11
Transportation......................................................................................................................12
Internal audits.......................................................................................................................12
Conclusion................................................................................................................................13
Reference list............................................................................................................................14
Table of Contents
Introduction................................................................................................................................3
Media Devices Company: An Overview and related issues......................................................4
Logistic management strategy....................................................................................................5
The segmented supply chain..................................................................................................5
The direct distribution model.................................................................................................6
Adding shared services..........................................................................................................8
The outsourcing model...........................................................................................................9
Benefits of 3PL....................................................................................................................10
Personnel and staff training..................................................................................................10
Key personnel...................................................................................................................10
Training............................................................................................................................11
Change control.....................................................................................................................11
Transportation......................................................................................................................12
Internal audits.......................................................................................................................12
Conclusion................................................................................................................................13
Reference list............................................................................................................................14
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
3THE MEDICAL DEVICES COMPANY
Introduction
“The medical technology sector (also known as the medical device sector) is
weathering a perfect storm, caused by three concurrent trends: the move toward value-based
healthcare, cost/margin pressures and increasing regulatory/compliance requirements” (Ernst
and Young 2013). “In particular, the industry must become far more efficient, and that
requires transformational strategies for managing the supply chain as a whole”.
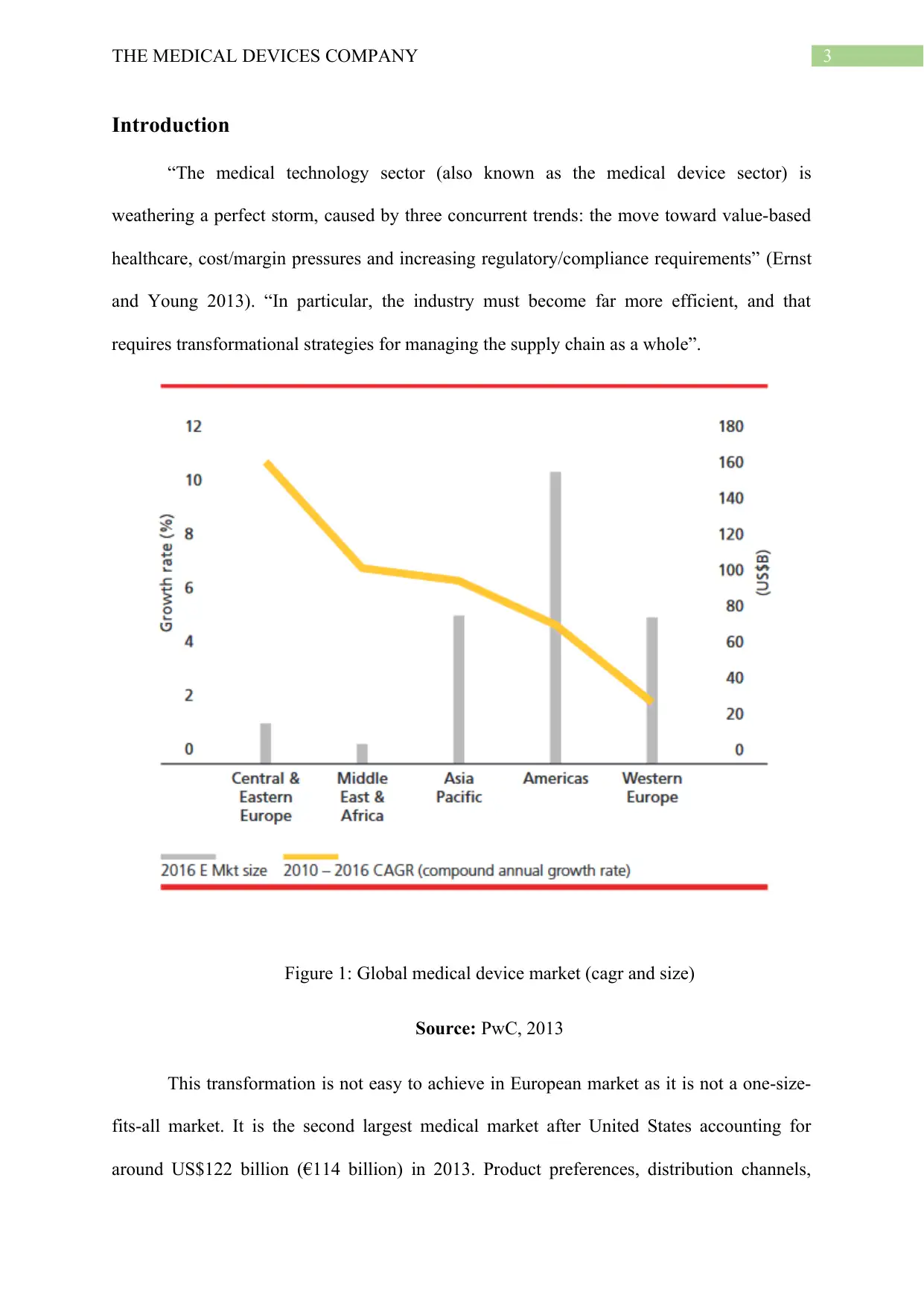
Figure 1: Global medical device market (cagr and size)
Source: PwC, 2013
This transformation is not easy to achieve in European market as it is not a one-size-
fits-all market. It is the second largest medical market after United States accounting for
around US$122 billion (€114 billion) in 2013. Product preferences, distribution channels,
Introduction
“The medical technology sector (also known as the medical device sector) is
weathering a perfect storm, caused by three concurrent trends: the move toward value-based
healthcare, cost/margin pressures and increasing regulatory/compliance requirements” (Ernst
and Young 2013). “In particular, the industry must become far more efficient, and that
requires transformational strategies for managing the supply chain as a whole”.
Figure 1: Global medical device market (cagr and size)
Source: PwC, 2013
This transformation is not easy to achieve in European market as it is not a one-size-
fits-all market. It is the second largest medical market after United States accounting for
around US$122 billion (€114 billion) in 2013. Product preferences, distribution channels,
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4THE MEDICAL DEVICES COMPANY
regulations and payer mechanisms widely varies from country to country. “There are
significant differences in how products are being secured and distributed, as well as the
nature of stakeholders involved in the sales and distribution process,” as said by Luiz
Moreira. European medical market is facing intensive pressure and momentum in this
competitive market. New complexities and costs are getting incorporated into the supply
chain when pressure from patients, governments, healthcare providers and payers to reduce
costs has never increased.
This paper explore in two aspects. Firstly, the current trend in the medical device
company and secondly to explore the logistics management strategies that can enable the
company to become successful, and/or will be necessary for the company to
maintain/improve its success in the future.
Media Devices Company: An Overview and related issues
Media devices Company (MDC) is a renowned multinational company that
manufactures advanced medical devices which are used in the operating room by the
surgeons. It also has a flexible distribution network for the delivery of its products. The
devices made by MDC are generally expensive with pricing starting from €2000 onwards.
The manufacturing process is carried out in two plants, one in Poland and the other in Ireland
for the European market. Other peripherals and devices are also sold in the brand name of
MDC, which initially is transported to the two manufacturing plants and after that it is
dispersed to the next levels of the Media Devices Company (MDC) supply chain. The
manufactured goods and products are firstly transported to 15 warehouses that are situated in
different parts of the Europe. These warehouses (15) acts as Hubs and distributes products to
other 40 warehouses that are mainly located in the urban areas of Europe. And, finally
regulations and payer mechanisms widely varies from country to country. “There are
significant differences in how products are being secured and distributed, as well as the
nature of stakeholders involved in the sales and distribution process,” as said by Luiz
Moreira. European medical market is facing intensive pressure and momentum in this
competitive market. New complexities and costs are getting incorporated into the supply
chain when pressure from patients, governments, healthcare providers and payers to reduce
costs has never increased.
This paper explore in two aspects. Firstly, the current trend in the medical device
company and secondly to explore the logistics management strategies that can enable the
company to become successful, and/or will be necessary for the company to
maintain/improve its success in the future.
Media Devices Company: An Overview and related issues
Media devices Company (MDC) is a renowned multinational company that
manufactures advanced medical devices which are used in the operating room by the
surgeons. It also has a flexible distribution network for the delivery of its products. The
devices made by MDC are generally expensive with pricing starting from €2000 onwards.
The manufacturing process is carried out in two plants, one in Poland and the other in Ireland
for the European market. Other peripherals and devices are also sold in the brand name of
MDC, which initially is transported to the two manufacturing plants and after that it is
dispersed to the next levels of the Media Devices Company (MDC) supply chain. The
manufactured goods and products are firstly transported to 15 warehouses that are situated in
different parts of the Europe. These warehouses (15) acts as Hubs and distributes products to
other 40 warehouses that are mainly located in the urban areas of Europe. And, finally

5THE MEDICAL DEVICES COMPANY
distribution agents and sales representatives draw their inventory from these 40 warehouses
to sell products to their customers.
Due to the advancement in medical technology and the increasing competition in the
business market, MDC has to cope with many challenges. Customers (like hospitals) are
demanding for improved and seamless services along with wide range of available choices
(products) to choose from. They also want lower levels of stock holding so that it will be
easier for them in the long run. Many customers demands that various alternatives of a
particular device should be made available to them and also they can pay only for the devices
that are being used in the operation. Many companies are offering such solutions thereby
increasing competition in the marketplace. There has been a steady fall in the inventory
turnover of the MDC and has reached to five turns per year (around 10 is the industry norm)
thereby increasing the inventory level of the system. Furthermore, product obsolescence has
also cropped up. Some cases need urgent requirement of specific devices for the surgery to
take place, so there is a need to optimise inventory as company is running out of stock at
various stages of supply chain.
Logistic management strategy
According to the statistics, medical device manufacturers stock inventory for more than
150 days as compared to automotive and speedy consumer goods that holds stock for 2-3
weeks and 70 days respectively. This wastage in the supply chain has to be curbed as a
paradigm shift can be seen towards pricing based on value and new government policy. The
logistic strategy of the MDCs must include decisions like:
Product value as a key factor in segmenting logistics and supply chain
Establishing a direct-to-consumer channel
Develop shared-use distribution centres
distribution agents and sales representatives draw their inventory from these 40 warehouses
to sell products to their customers.
Due to the advancement in medical technology and the increasing competition in the
business market, MDC has to cope with many challenges. Customers (like hospitals) are
demanding for improved and seamless services along with wide range of available choices
(products) to choose from. They also want lower levels of stock holding so that it will be
easier for them in the long run. Many customers demands that various alternatives of a
particular device should be made available to them and also they can pay only for the devices
that are being used in the operation. Many companies are offering such solutions thereby
increasing competition in the marketplace. There has been a steady fall in the inventory
turnover of the MDC and has reached to five turns per year (around 10 is the industry norm)
thereby increasing the inventory level of the system. Furthermore, product obsolescence has
also cropped up. Some cases need urgent requirement of specific devices for the surgery to
take place, so there is a need to optimise inventory as company is running out of stock at
various stages of supply chain.
Logistic management strategy
According to the statistics, medical device manufacturers stock inventory for more than
150 days as compared to automotive and speedy consumer goods that holds stock for 2-3
weeks and 70 days respectively. This wastage in the supply chain has to be curbed as a
paradigm shift can be seen towards pricing based on value and new government policy. The
logistic strategy of the MDCs must include decisions like:
Product value as a key factor in segmenting logistics and supply chain
Establishing a direct-to-consumer channel
Develop shared-use distribution centres
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6THE MEDICAL DEVICES COMPANY
Assigning outbound logistics to third party
The segmented supply chain
The costing of the supply chain structures should be aligned according to the
characteristics of different customer categories and product. Therefore, manufacturers are
nowadays fostering segmentation in their supply chain. Segmentation includes restructuring
of the warehousing and transportation system according to the type of medical devices
(Plenert 2014). Oceans and roads can be used to transport goods of high volumes that are of
low value. These products can also be channelized through local distribution centres & cross-
dock facilities which are located in proximity to end market. High-end or expensive medical
devices can be shipped through faster modes of transportation which includes premium
services like express or air services and distributed to hospitals via single regional or global
distribution hub. Critical goods and products can be stored in a central air hubs of European
airport for easy distribution in case of urgencies. An alternative approach can also be made by
utilizing small shared warehouses that are close to usage point so urgent needs of the
hospitals can be met. Both these approaches can be used for inventory control.
Analysts believe that by 2025, various medical devices company will adopt these
segmentation process by restructuring their supply chains in accordance with below
mentioned categories:
Products of high value
Innovative standard equipments
Generic or low-tech devices
Low value medical devices
Assigning outbound logistics to third party
The segmented supply chain
The costing of the supply chain structures should be aligned according to the
characteristics of different customer categories and product. Therefore, manufacturers are
nowadays fostering segmentation in their supply chain. Segmentation includes restructuring
of the warehousing and transportation system according to the type of medical devices
(Plenert 2014). Oceans and roads can be used to transport goods of high volumes that are of
low value. These products can also be channelized through local distribution centres & cross-
dock facilities which are located in proximity to end market. High-end or expensive medical
devices can be shipped through faster modes of transportation which includes premium
services like express or air services and distributed to hospitals via single regional or global
distribution hub. Critical goods and products can be stored in a central air hubs of European
airport for easy distribution in case of urgencies. An alternative approach can also be made by
utilizing small shared warehouses that are close to usage point so urgent needs of the
hospitals can be met. Both these approaches can be used for inventory control.
Analysts believe that by 2025, various medical devices company will adopt these
segmentation process by restructuring their supply chains in accordance with below
mentioned categories:
Products of high value
Innovative standard equipments
Generic or low-tech devices
Low value medical devices
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7THE MEDICAL DEVICES COMPANY
The direct distribution model
The manufacturers can deliver products direct to customers to ensure profits and low
cost. This can be considered as an alternate approach to the distributor-intermediary model.
The direct service to customers depends on the security needs, product cost & total no. of
final distribution points. “An increasing number of manufacturers are looking at serving more
of their customers directly, rather than through distributors or agents, especially in developing
Eastern European countries. There is a mutual interest between manufacturers and their
customers (healthcare providers) to create these direct bonds. Hospitals can benefit from
product knowledge transfer coming directly from the people who make the products.
Manufacturers can collect more accurate sales and customer usage information, and get direct
customer feedback about product performance and service satisfaction.”
Figure 2: Old insourced distribution model
Source: DHL Supply Chain, 2015
The direct distribution model
The manufacturers can deliver products direct to customers to ensure profits and low
cost. This can be considered as an alternate approach to the distributor-intermediary model.
The direct service to customers depends on the security needs, product cost & total no. of
final distribution points. “An increasing number of manufacturers are looking at serving more
of their customers directly, rather than through distributors or agents, especially in developing
Eastern European countries. There is a mutual interest between manufacturers and their
customers (healthcare providers) to create these direct bonds. Hospitals can benefit from
product knowledge transfer coming directly from the people who make the products.
Manufacturers can collect more accurate sales and customer usage information, and get direct
customer feedback about product performance and service satisfaction.”
Figure 2: Old insourced distribution model
Source: DHL Supply Chain, 2015
8THE MEDICAL DEVICES COMPANY
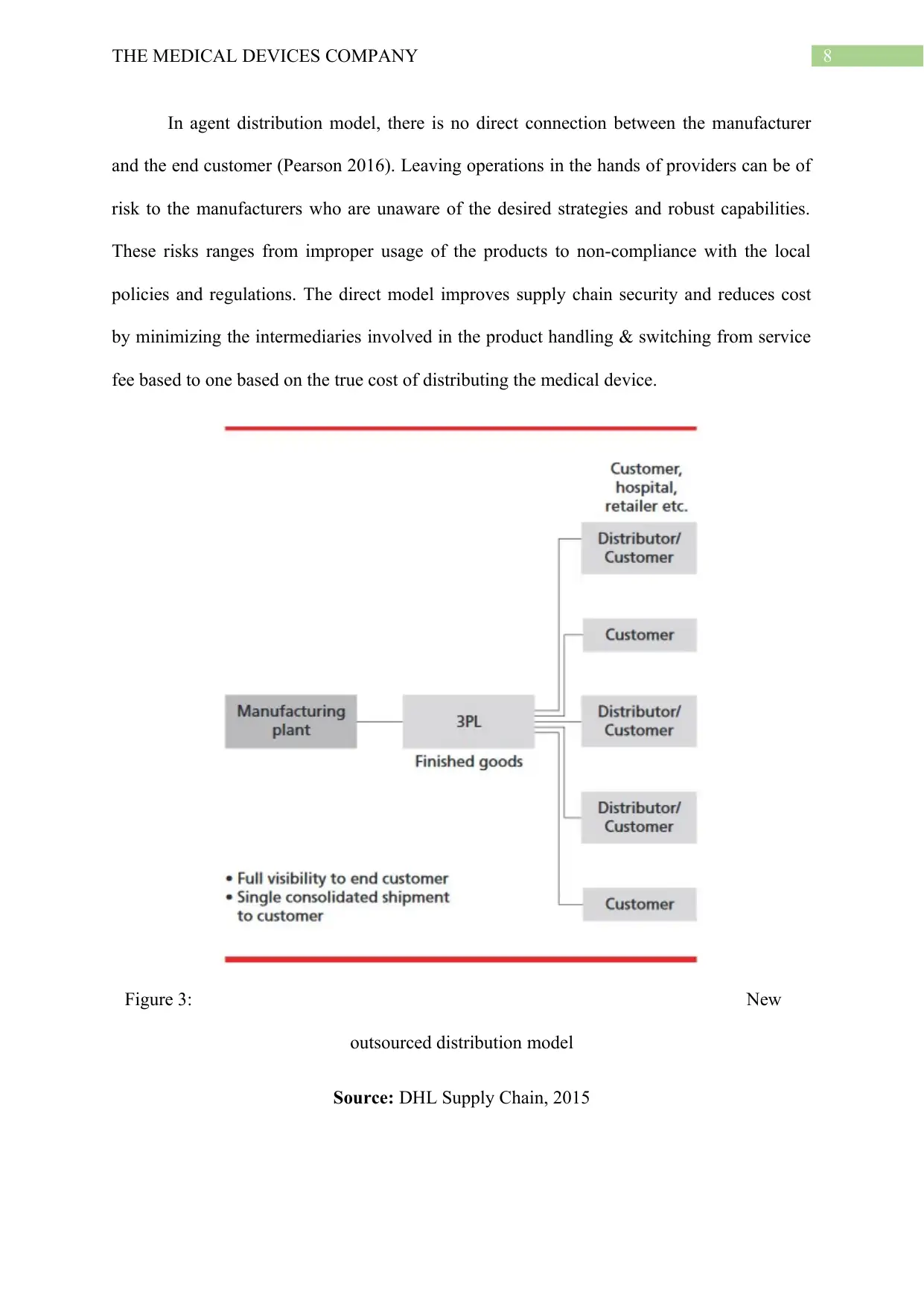
In agent distribution model, there is no direct connection between the manufacturer
and the end customer (Pearson 2016). Leaving operations in the hands of providers can be of
risk to the manufacturers who are unaware of the desired strategies and robust capabilities.
These risks ranges from improper usage of the products to non-compliance with the local
policies and regulations. The direct model improves supply chain security and reduces cost
by minimizing the intermediaries involved in the product handling & switching from service
fee based to one based on the true cost of distributing the medical device.
Figure 3: New
outsourced distribution model
Source: DHL Supply Chain, 2015
In agent distribution model, there is no direct connection between the manufacturer
and the end customer (Pearson 2016). Leaving operations in the hands of providers can be of
risk to the manufacturers who are unaware of the desired strategies and robust capabilities.
These risks ranges from improper usage of the products to non-compliance with the local
policies and regulations. The direct model improves supply chain security and reduces cost
by minimizing the intermediaries involved in the product handling & switching from service
fee based to one based on the true cost of distributing the medical device.
Figure 3: New
outsourced distribution model
Source: DHL Supply Chain, 2015
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9THE MEDICAL DEVICES COMPANY
Adding shared services
Developing a shared services operating model can also reduce costs and optimize
supply chain. Typically, medical device companies provides services to same kind of
customers (hospitals, clinics etc.) due to which they run parallel supply chains in order to
meet customers’ demands. This redundancy and duplication of supply chains leads to
unnecessary costs for customers as well as manufacturers.
In the shared services operating model, various manufacturers accommodate their
products under a single 3PL with wide inter related facilities and distribution channel. The
sharing of these warehousing systems, transportation and distribution system ensures smooth
running of the supply chain for the customers and manufacturers.
The outsourcing model
The outsourcing of logistics operations and activities into the hands of 3PL with expertise
in handling supply chain activities will ease up the manufacturers to focus on producing and
developing medical devices (Giri and Sarkar 2017). 3PL knowledge, portfolio and
infrastructure ensures the flexibility of the supply chain and logistics.
This kind of partnership maximises productivity as the manufacturer is flexible enough to
analyse and assess the complexities of the European market viz. customer requirements,
products, infrastructure and regulations. A 3PL can provide:
Solutions that fulfils the demands and needs of country and markets thereby providing
a better solution than the one-size-fits-all distribution solution
Expertise to ensure tracking, packaging and labelling of medical devices who are also
well aware of the government’s regulations.
Efficient transportation and warehousing management solutions along with the
futuristic information systems.
Adding shared services
Developing a shared services operating model can also reduce costs and optimize
supply chain. Typically, medical device companies provides services to same kind of
customers (hospitals, clinics etc.) due to which they run parallel supply chains in order to
meet customers’ demands. This redundancy and duplication of supply chains leads to
unnecessary costs for customers as well as manufacturers.
In the shared services operating model, various manufacturers accommodate their
products under a single 3PL with wide inter related facilities and distribution channel. The
sharing of these warehousing systems, transportation and distribution system ensures smooth
running of the supply chain for the customers and manufacturers.
The outsourcing model
The outsourcing of logistics operations and activities into the hands of 3PL with expertise
in handling supply chain activities will ease up the manufacturers to focus on producing and
developing medical devices (Giri and Sarkar 2017). 3PL knowledge, portfolio and
infrastructure ensures the flexibility of the supply chain and logistics.
This kind of partnership maximises productivity as the manufacturer is flexible enough to
analyse and assess the complexities of the European market viz. customer requirements,
products, infrastructure and regulations. A 3PL can provide:
Solutions that fulfils the demands and needs of country and markets thereby providing
a better solution than the one-size-fits-all distribution solution
Expertise to ensure tracking, packaging and labelling of medical devices who are also
well aware of the government’s regulations.
Efficient transportation and warehousing management solutions along with the
futuristic information systems.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10THE MEDICAL DEVICES COMPANY
Flexibility and capabilities to support business change and market expansion.
There are many benefits that the manufacturer enjoys by partnering up with the 3PL. This
strong relationship ensures cost savings which is obtained by optimization of supply chain.
Optimizing helps a lot in this increasing cost pressure in these unprecedented business
sectors. “The different areas of saving potential can range as high as 20 percent, depending
on the solution,” as reported by Susanne Amholt. 3PL plays a crucial role in the management
of supply chain. It ensures the efficient end-to-end distribution of the products by looking
into the visibility and control of the product flow, managing distributors and logistics service
providers, tracking and coordinating deliveries to make sure of the accurate, effective and
timely delivery of the products to the ultimate customers.
Benefits of 3PL
Cost efficiency is improved
Cost effective, consistent and reliable delivery and transportation
Network flexibility
Service flexibility
Increased visibility of the supply chain (end-to-end)
Regular improvement process
Demonstrates best practices of supply chain
Sophisticated condition management, smooth track and trace
Personnel and staff training
Adequate training and experience should be provided to the personnel so that they can
properly perform their assign duties. Training should be well recorded and documented. Role
profiles and reporting structures should be properly tailored.
Flexibility and capabilities to support business change and market expansion.
There are many benefits that the manufacturer enjoys by partnering up with the 3PL. This
strong relationship ensures cost savings which is obtained by optimization of supply chain.
Optimizing helps a lot in this increasing cost pressure in these unprecedented business
sectors. “The different areas of saving potential can range as high as 20 percent, depending
on the solution,” as reported by Susanne Amholt. 3PL plays a crucial role in the management
of supply chain. It ensures the efficient end-to-end distribution of the products by looking
into the visibility and control of the product flow, managing distributors and logistics service
providers, tracking and coordinating deliveries to make sure of the accurate, effective and
timely delivery of the products to the ultimate customers.
Benefits of 3PL
Cost efficiency is improved
Cost effective, consistent and reliable delivery and transportation
Network flexibility
Service flexibility
Increased visibility of the supply chain (end-to-end)
Regular improvement process
Demonstrates best practices of supply chain
Sophisticated condition management, smooth track and trace
Personnel and staff training
Adequate training and experience should be provided to the personnel so that they can
properly perform their assign duties. Training should be well recorded and documented. Role
profiles and reporting structures should be properly tailored.

11THE MEDICAL DEVICES COMPANY
Key personnel
A qualified personnel can be assigned who can act as a mediator between the
distributor and the concerned authority. This person should be made responsible for the
effectiveness of the quality system. Other duties includes handling of safety notices and
product recalls, segregation, storage and release of stocks.
Training
Training is a very important component in a quality system. A training plan should be
developed in order to make staff member aware of their job responsibility. Before taking any
responsibility it is quite important that significance of training should be possessed on. The
staff members is required to show the required competencies. On the other hand again it
becomes important to record as well as well maintain the evidence with respect to specific
effects. It becomes next important to maintain in detail about each and every training events
and it is further required to be properly documented. This should include-in details about
training, place where the training will take place as well as total time period required, the
person providing the training and also it should be needed to assure about the trainee that
whether he has achieved with his necessary requirement of competence. The achievement of
required level of competence should be related to the aims and objectives in terms of event
conducted for training. It becomes further necessary to include the revised number of
documents related to training. It is necessary and important for both the trainer as well as
trainee to conclude for training related records. Training must include- specified
responsibilities related to the personnel, any kind of requirement that can be related to
storage, labelling checks and process of all complaints as well as necessary principle
requirements that are related to the documents for guidance (Does, Nestler and Kewley
2013).
Key personnel
A qualified personnel can be assigned who can act as a mediator between the
distributor and the concerned authority. This person should be made responsible for the
effectiveness of the quality system. Other duties includes handling of safety notices and
product recalls, segregation, storage and release of stocks.
Training
Training is a very important component in a quality system. A training plan should be
developed in order to make staff member aware of their job responsibility. Before taking any
responsibility it is quite important that significance of training should be possessed on. The
staff members is required to show the required competencies. On the other hand again it
becomes important to record as well as well maintain the evidence with respect to specific
effects. It becomes next important to maintain in detail about each and every training events
and it is further required to be properly documented. This should include-in details about
training, place where the training will take place as well as total time period required, the
person providing the training and also it should be needed to assure about the trainee that
whether he has achieved with his necessary requirement of competence. The achievement of
required level of competence should be related to the aims and objectives in terms of event
conducted for training. It becomes further necessary to include the revised number of
documents related to training. It is necessary and important for both the trainer as well as
trainee to conclude for training related records. Training must include- specified
responsibilities related to the personnel, any kind of requirement that can be related to
storage, labelling checks and process of all complaints as well as necessary principle
requirements that are related to the documents for guidance (Does, Nestler and Kewley
2013).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





