Medication Information Report: Detailed Case Study Analysis Solution
VerifiedAdded on 2022/08/22
|3
|644
|29
Report
AI Summary
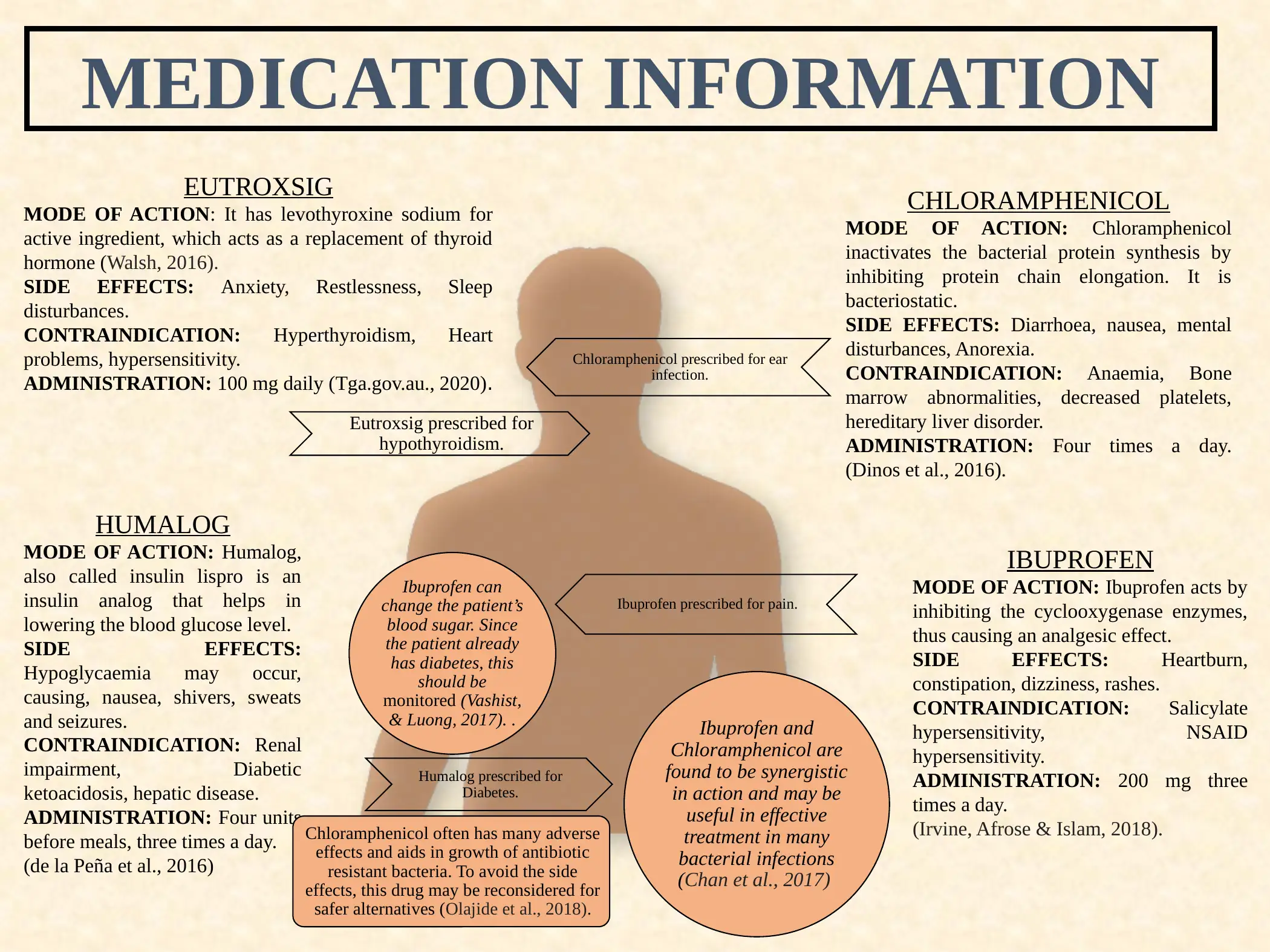
This report presents detailed medication information for a specific case study involving a 25-year-old single mother with hypothyroidism, Type 1 Diabetes, and an ear infection. The report covers four medications: Eutroxsig (levothyroxine sodium), Chloramphenicol, Ibuprofen, and Humalog (insulin lispro). For each medication, the report outlines the mode of action, common side effects, contraindications, and recommended administration guidelines. References are provided to support the information. The assignment is a response to a case study where the patient is prescribed these medications. The report is aimed at providing concise and relevant information about the drugs used in the case study.
1 out of 3



![[object Object]](/_next/static/media/star-bottom.7253800d.svg)