Report on Legislation, Codes & Policies for Medication Handling
VerifiedAdded on 2023/06/12
|3
|767
|117
Report
AI Summary
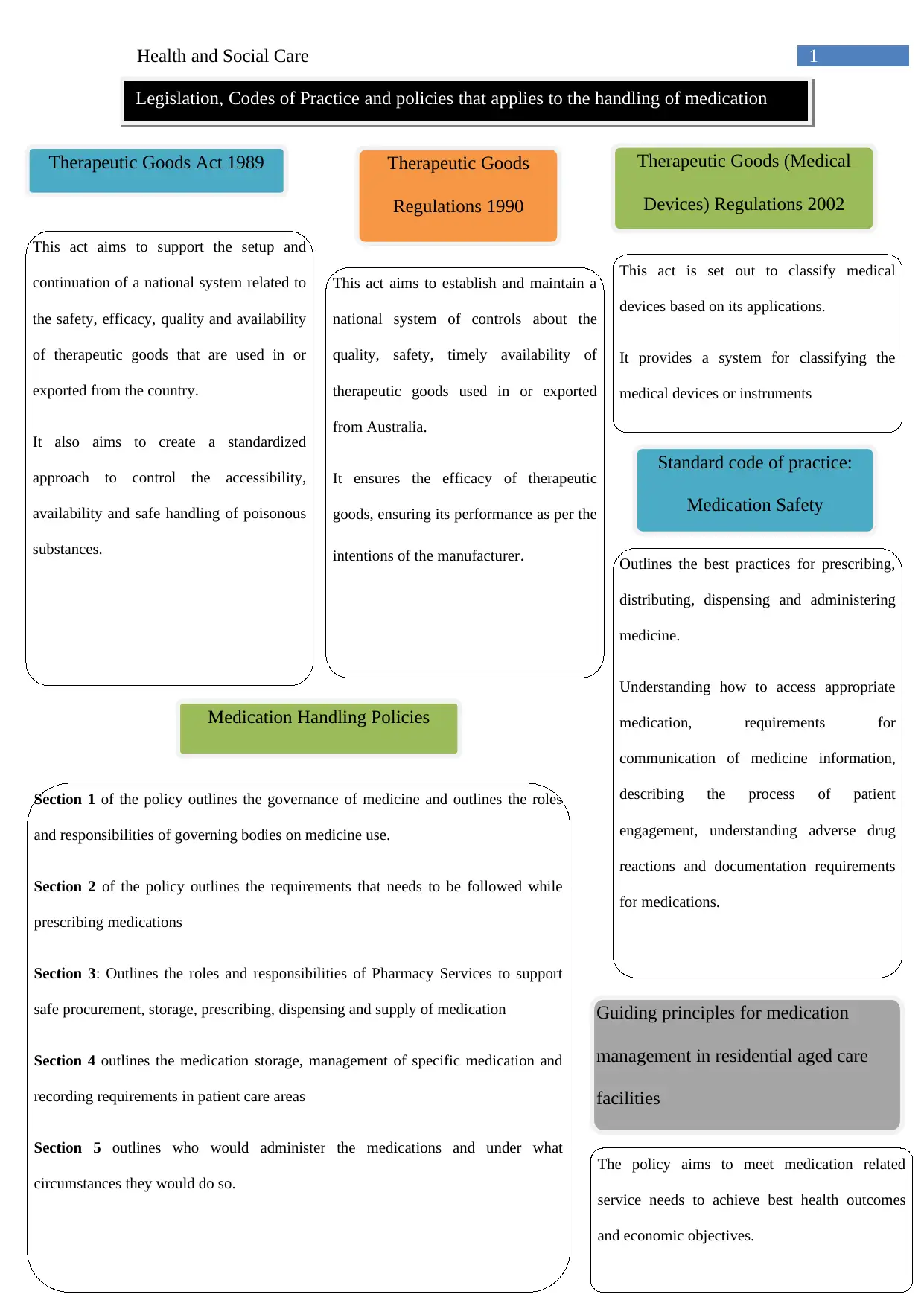
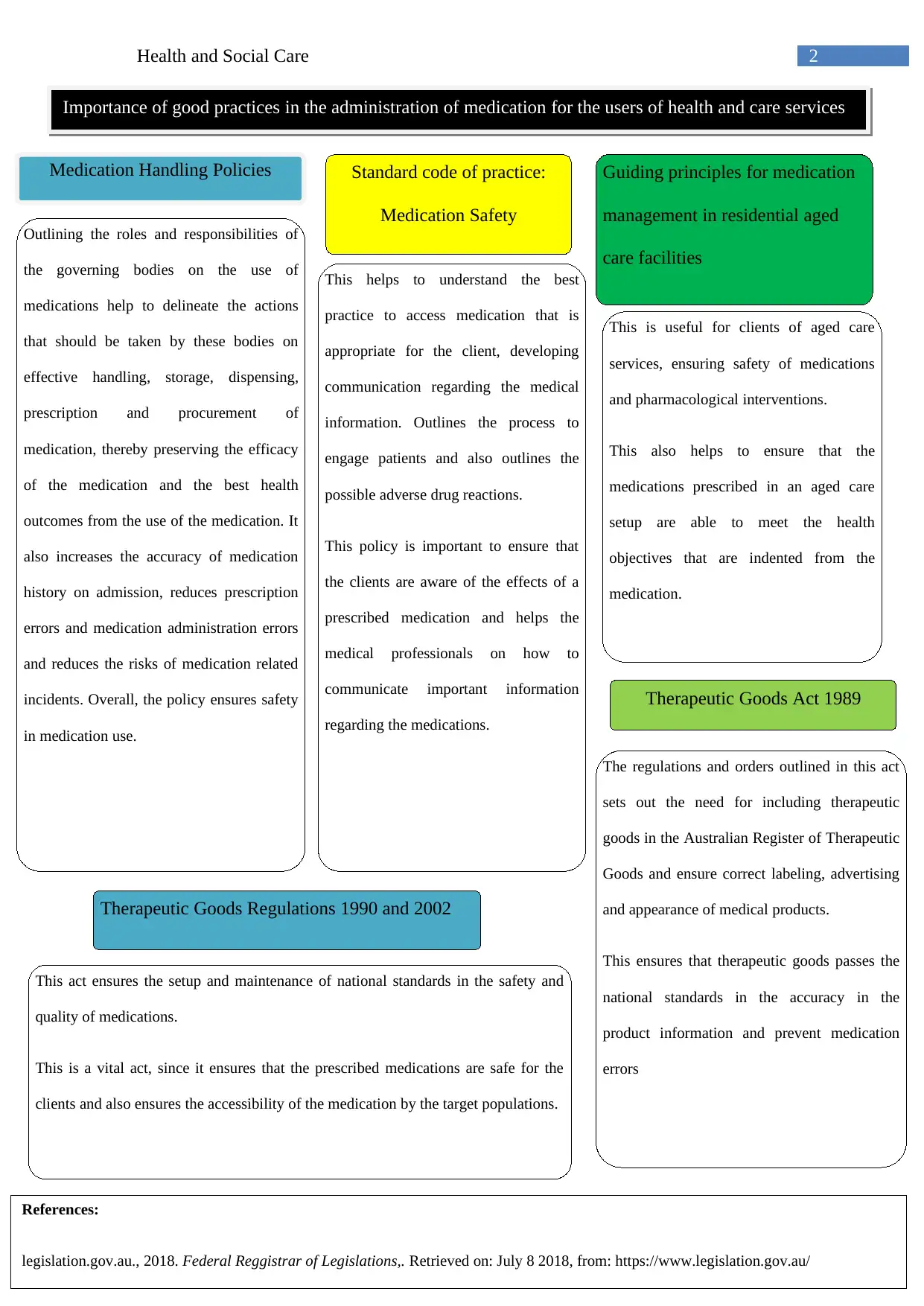
This report provides an overview of the legislation, codes of practice, and policies that apply to the handling of medication in health and social care settings. It discusses the Therapeutic Goods Act 1989 and related regulations, highlighting their role in ensuring the safety, efficacy, and quality of therapeutic goods. The report also examines the Standard Code of Practice for Medication Safety, outlining best practices for prescribing, distributing, dispensing, and administering medicine. Furthermore, it delves into medication handling policies, emphasizing the roles and responsibilities of governing bodies, pharmacy services, and healthcare professionals in ensuring safe medication management. The report concludes by underscoring the importance of good practices in medication administration for the users of health and care services, emphasizing patient safety, accuracy, and adherence to regulations.
1 out of 3








![[object Object]](/_next/static/media/star-bottom.7253800d.svg)