Report: Metabolic Engineering of Omega 3 Fatty Acids in E. coli
VerifiedAdded on 2023/04/24
|7
|1552
|100
Report
AI Summary
This report delves into the metabolic engineering of omega 3 fatty acids in E. coli, utilizing lignocellulosic hydrolysate as a base. It begins by outlining the normal pathway of fatty acid production in E. coli, highlighting the enzymes and processes involved, such as the formation of malonyl-CoA and the role of acyl carrier protein (ACP). The report then explores various modification strategies to enhance fatty acid synthesis, including increasing export rates, regulating membrane saturation, addressing metabolic bottlenecks, and conducting structural studies. It identifies the modification of ΔfadE with TesA′ thioesterase as a promising route, citing studies that have achieved significant theoretical yields of fatty acids through this method. The report concludes that while modifying fatty acid pathways in E. coli holds great potential for applications like biofuel production, further research is needed to optimize the process and achieve consistent, high yields. The report references several studies and includes figures illustrating biosynthesis and the working mechanism of designed promoters.

Running head: METABOLIC ENGINEERING IN E.COLI
The metabolic engineering of omega 3 fatty acids in E. coli from lignocellulosic hydrolysate
Name of the Student
Name of the University
Author’s Note:
The metabolic engineering of omega 3 fatty acids in E. coli from lignocellulosic hydrolysate
Name of the Student
Name of the University
Author’s Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1METABOLIC ENGINEERING IN E.COLI
Introduction
Fatty acid synthesis in prokaryotic and eukaryotic organisms is one of the most
omnipresent pathways. Synthesis of fatty acids is vital to this organisms as it is precursors for
various building blocks like sterols, sphingolipids, secondary metabolites, and phospholipids.
This pathway was developed in very early stage of evolution but it is dogmatically conserved
amongst the kingdom of life. Additionally, proper understanding and modification of this
pathway can have significant effect on the area like bio- diesel production (Tee et al. 2014).
Therefore, in this report, fatty acid production pathway in E. coliand modification process of
this pathway will be discussed.
Discussion
Pathway of fatty acid production in E. coli:
The first stage of the fatty acid synthesis is the formation of malonyl-CoA which is formed by
carboxylation of acetyl-CoA. This process cost one ATP. Then, ACP or acyl carrier protein
exchanged the Coenzyme A in the malonyl-CoA and became malonyl-ACP. The action of
this ACP or acyl carrier protein is to prevent this growing fatty acid chain from being
degraded or used in anabolic reactions. Condensation of malonyl-ACP with acetyl-CoA starts
the first steps of the cycle of fatty acid synthesis. The by- products of this steps are free
coenzyme A, hydrogencarbonate, and acetoacetyl-ACP. Acetoacetyl-ACP then reduced to 3-
hydroxybutyryl-ACP which is then converted to 2-butenoyl-ACP by dehydration. 2-butenoyl-
ACP further reduced to butyryl-ACP. The next step of this cycles begins with butyryl-ACP
and its condensation with malonyl-ACP. The synthesis of the fatty acids continues until it
reaches a certain length. After reaching that required chain length, membrane synthesis
performed using the acyl-ACP. Reduction equivalent required by these two reduction step are
derived from NADPH or nicotinamide adenine dinucleotide (Schweizer and Hofmann 2004).
Introduction
Fatty acid synthesis in prokaryotic and eukaryotic organisms is one of the most
omnipresent pathways. Synthesis of fatty acids is vital to this organisms as it is precursors for
various building blocks like sterols, sphingolipids, secondary metabolites, and phospholipids.
This pathway was developed in very early stage of evolution but it is dogmatically conserved
amongst the kingdom of life. Additionally, proper understanding and modification of this
pathway can have significant effect on the area like bio- diesel production (Tee et al. 2014).
Therefore, in this report, fatty acid production pathway in E. coliand modification process of
this pathway will be discussed.
Discussion
Pathway of fatty acid production in E. coli:
The first stage of the fatty acid synthesis is the formation of malonyl-CoA which is formed by
carboxylation of acetyl-CoA. This process cost one ATP. Then, ACP or acyl carrier protein
exchanged the Coenzyme A in the malonyl-CoA and became malonyl-ACP. The action of
this ACP or acyl carrier protein is to prevent this growing fatty acid chain from being
degraded or used in anabolic reactions. Condensation of malonyl-ACP with acetyl-CoA starts
the first steps of the cycle of fatty acid synthesis. The by- products of this steps are free
coenzyme A, hydrogencarbonate, and acetoacetyl-ACP. Acetoacetyl-ACP then reduced to 3-
hydroxybutyryl-ACP which is then converted to 2-butenoyl-ACP by dehydration. 2-butenoyl-
ACP further reduced to butyryl-ACP. The next step of this cycles begins with butyryl-ACP
and its condensation with malonyl-ACP. The synthesis of the fatty acids continues until it
reaches a certain length. After reaching that required chain length, membrane synthesis
performed using the acyl-ACP. Reduction equivalent required by these two reduction step are
derived from NADPH or nicotinamide adenine dinucleotide (Schweizer and Hofmann 2004).
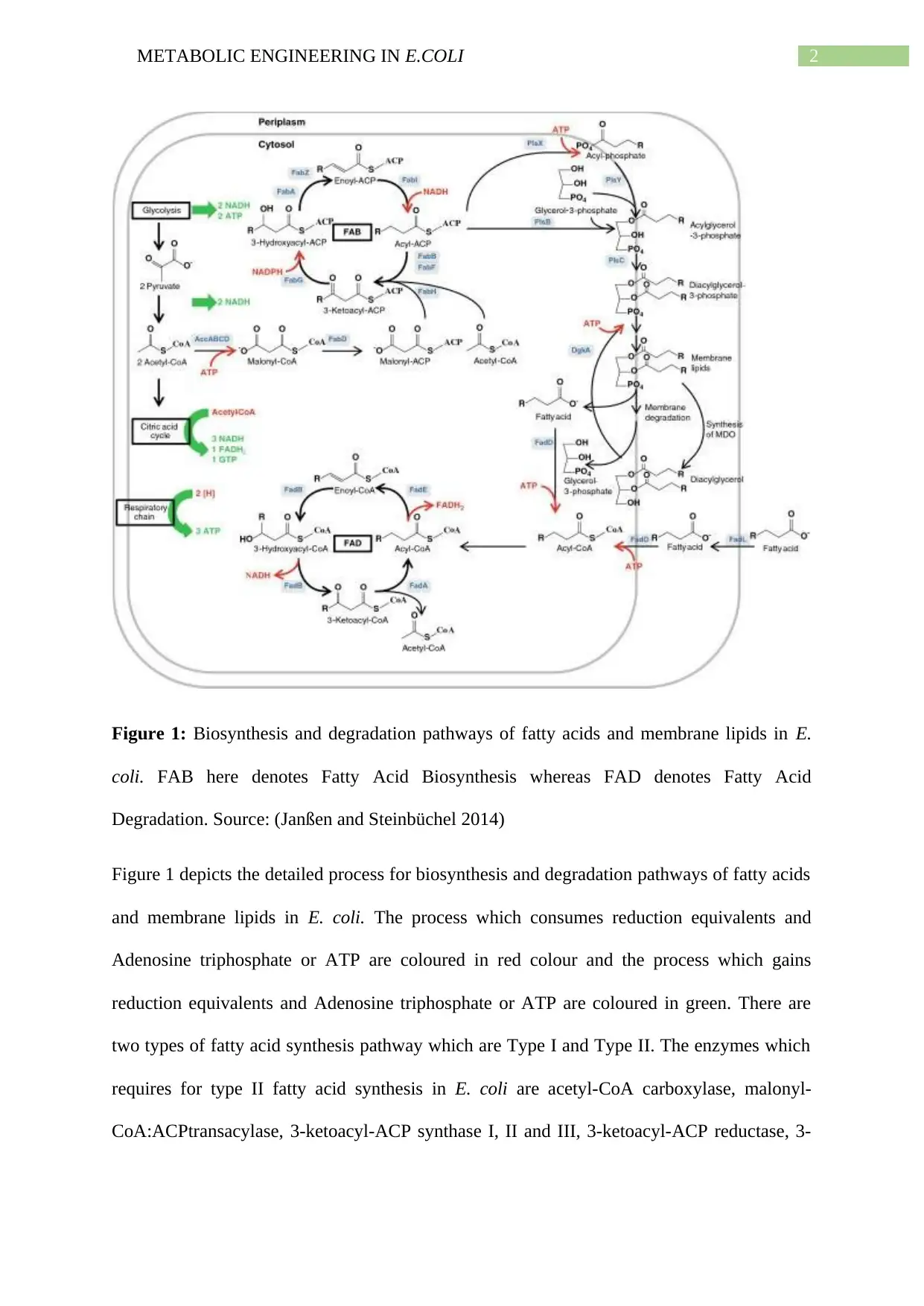
2METABOLIC ENGINEERING IN E.COLI
Figure 1: Biosynthesis and degradation pathways of fatty acids and membrane lipids in E.
coli. FAB here denotes Fatty Acid Biosynthesis whereas FAD denotes Fatty Acid
Degradation. Source: (Janßen and Steinbüchel 2014)
Figure 1 depicts the detailed process for biosynthesis and degradation pathways of fatty acids
and membrane lipids in E. coli. The process which consumes reduction equivalents and
Adenosine triphosphate or ATP are coloured in red colour and the process which gains
reduction equivalents and Adenosine triphosphate or ATP are coloured in green. There are
two types of fatty acid synthesis pathway which are Type I and Type II. The enzymes which
requires for type II fatty acid synthesis in E. coli are acetyl-CoA carboxylase, malonyl-
CoA:ACPtransacylase, 3-ketoacyl-ACP synthase I, II and III, 3-ketoacyl-ACP reductase, 3-
Figure 1: Biosynthesis and degradation pathways of fatty acids and membrane lipids in E.
coli. FAB here denotes Fatty Acid Biosynthesis whereas FAD denotes Fatty Acid
Degradation. Source: (Janßen and Steinbüchel 2014)
Figure 1 depicts the detailed process for biosynthesis and degradation pathways of fatty acids
and membrane lipids in E. coli. The process which consumes reduction equivalents and
Adenosine triphosphate or ATP are coloured in red colour and the process which gains
reduction equivalents and Adenosine triphosphate or ATP are coloured in green. There are
two types of fatty acid synthesis pathway which are Type I and Type II. The enzymes which
requires for type II fatty acid synthesis in E. coli are acetyl-CoA carboxylase, malonyl-
CoA:ACPtransacylase, 3-ketoacyl-ACP synthase I, II and III, 3-ketoacyl-ACP reductase, 3-
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3METABOLIC ENGINEERING IN E.COLI
hydroxyacyl-ACP dehydrase, enoyl-ACP reductase, ACP, ACP synthase and ACP
phosphodiesterase (Jeon et al. 2012).
Process modification of omega -3- fatty acids synthesis in E. coli:
Four different modification directions can be taken to improve the fatty acid synthesis in E.
coli. These modification directions are namely a) Increase of export rates, b) Regulation of
membrane saturation, c) Addressing the regulatory and metabolic bottlenecks, and d)
Structural, genetic and biochemistry studies. From the fore mentioned methods, identification
of metabolic bottlenecks and optimal cultivation strategy design might be the best possible
option to generate maximum yield for fatty acid synthesis. The reason behind the selection of
this method is that this particular direction has the maximum chance to succeed in regard to
other methods. Lennen et al. (2011) have modified the actions of ΔfadD with BTE
thioesterase and have achieved a total theoretical yield of less than 40 per cent. They have
used K-12 MG1655 base strain for their experiment. In this study, the authors have modified
the functions of ΔfadE by deletion and noticed a change if decrement in the expression of
fabB and fabA which were expressed in BTE-expressing strains. According to the author this
might be the reason for the modified yield. Theoretical yield mentioned in this study means
that produced fatty acids were less than 40 per cent of total mass used weight by weight.
Another study has modified the actions of ΔfadL with TesA′ thioesterase in the base strain of
BL21 (DE3). The total theoretical yield in that study was reported at around 12 per cent (Liu
et al. 2012). The authors of this study have modified the gene expression of the base strain
BL21 (DE3) by deleting the enzyme fadL. They have reported that this deletion have resulted
in an overexpression of TesA′ thioesterase. However, they reported that this modification
does not enhance the end line fatty acid production. Youngquist et al. (2012) have modified
the base strain’s gene expression by deleting the ΔfadD, ΔfadE, and ΔfadAB enzyme. The
hydroxyacyl-ACP dehydrase, enoyl-ACP reductase, ACP, ACP synthase and ACP
phosphodiesterase (Jeon et al. 2012).
Process modification of omega -3- fatty acids synthesis in E. coli:
Four different modification directions can be taken to improve the fatty acid synthesis in E.
coli. These modification directions are namely a) Increase of export rates, b) Regulation of
membrane saturation, c) Addressing the regulatory and metabolic bottlenecks, and d)
Structural, genetic and biochemistry studies. From the fore mentioned methods, identification
of metabolic bottlenecks and optimal cultivation strategy design might be the best possible
option to generate maximum yield for fatty acid synthesis. The reason behind the selection of
this method is that this particular direction has the maximum chance to succeed in regard to
other methods. Lennen et al. (2011) have modified the actions of ΔfadD with BTE
thioesterase and have achieved a total theoretical yield of less than 40 per cent. They have
used K-12 MG1655 base strain for their experiment. In this study, the authors have modified
the functions of ΔfadE by deletion and noticed a change if decrement in the expression of
fabB and fabA which were expressed in BTE-expressing strains. According to the author this
might be the reason for the modified yield. Theoretical yield mentioned in this study means
that produced fatty acids were less than 40 per cent of total mass used weight by weight.
Another study has modified the actions of ΔfadL with TesA′ thioesterase in the base strain of
BL21 (DE3). The total theoretical yield in that study was reported at around 12 per cent (Liu
et al. 2012). The authors of this study have modified the gene expression of the base strain
BL21 (DE3) by deleting the enzyme fadL. They have reported that this deletion have resulted
in an overexpression of TesA′ thioesterase. However, they reported that this modification
does not enhance the end line fatty acid production. Youngquist et al. (2012) have modified
the base strain’s gene expression by deleting the ΔfadD, ΔfadE, and ΔfadAB enzyme. The
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
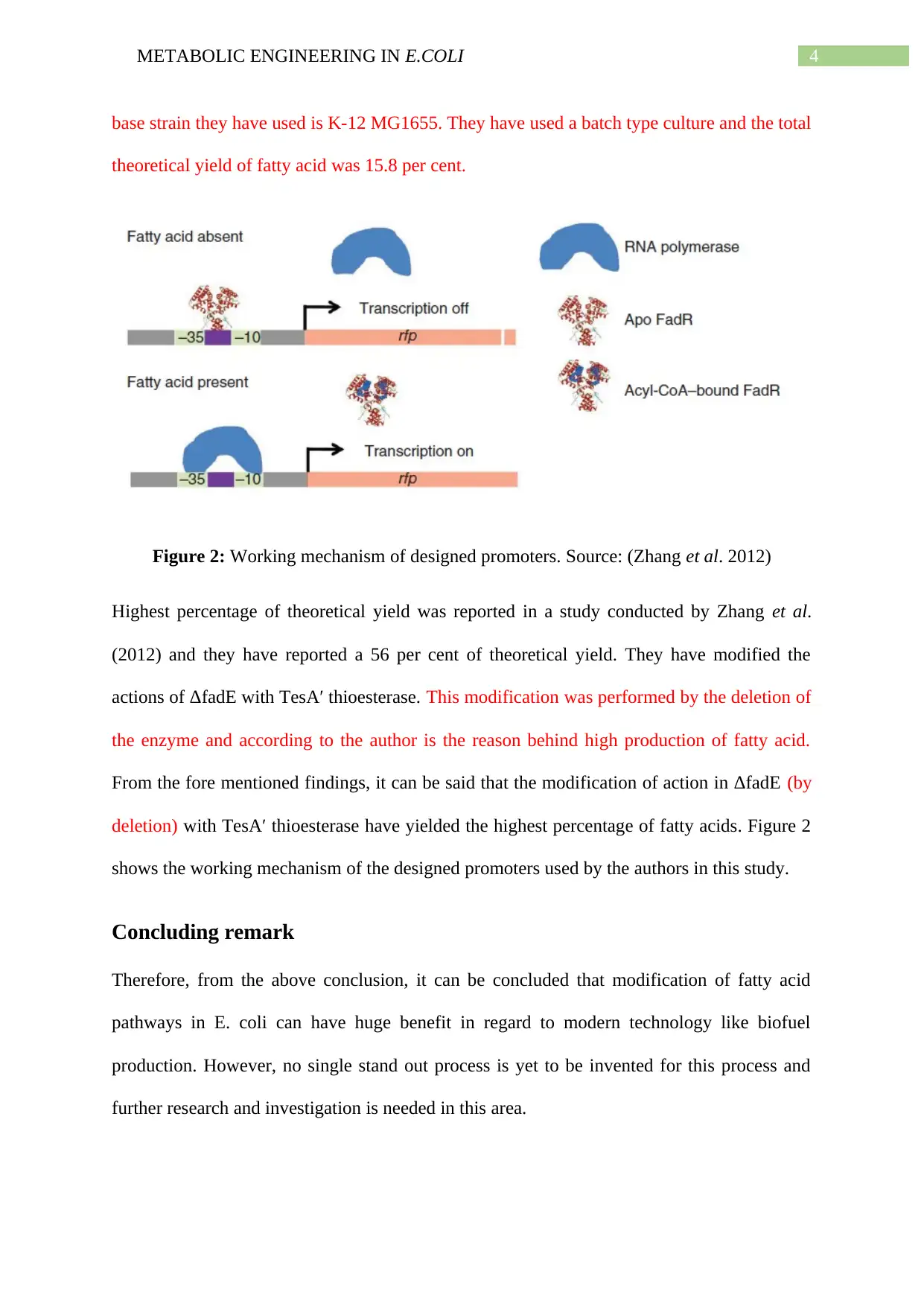
4METABOLIC ENGINEERING IN E.COLI
base strain they have used is K-12 MG1655. They have used a batch type culture and the total
theoretical yield of fatty acid was 15.8 per cent.
Figure 2: Working mechanism of designed promoters. Source: (Zhang et al. 2012)
Highest percentage of theoretical yield was reported in a study conducted by Zhang et al.
(2012) and they have reported a 56 per cent of theoretical yield. They have modified the
actions of ΔfadE with TesA′ thioesterase. This modification was performed by the deletion of
the enzyme and according to the author is the reason behind high production of fatty acid.
From the fore mentioned findings, it can be said that the modification of action in ΔfadE (by
deletion) with TesA′ thioesterase have yielded the highest percentage of fatty acids. Figure 2
shows the working mechanism of the designed promoters used by the authors in this study.
Concluding remark
Therefore, from the above conclusion, it can be concluded that modification of fatty acid
pathways in E. coli can have huge benefit in regard to modern technology like biofuel
production. However, no single stand out process is yet to be invented for this process and
further research and investigation is needed in this area.
base strain they have used is K-12 MG1655. They have used a batch type culture and the total
theoretical yield of fatty acid was 15.8 per cent.
Figure 2: Working mechanism of designed promoters. Source: (Zhang et al. 2012)
Highest percentage of theoretical yield was reported in a study conducted by Zhang et al.
(2012) and they have reported a 56 per cent of theoretical yield. They have modified the
actions of ΔfadE with TesA′ thioesterase. This modification was performed by the deletion of
the enzyme and according to the author is the reason behind high production of fatty acid.
From the fore mentioned findings, it can be said that the modification of action in ΔfadE (by
deletion) with TesA′ thioesterase have yielded the highest percentage of fatty acids. Figure 2
shows the working mechanism of the designed promoters used by the authors in this study.
Concluding remark
Therefore, from the above conclusion, it can be concluded that modification of fatty acid
pathways in E. coli can have huge benefit in regard to modern technology like biofuel
production. However, no single stand out process is yet to be invented for this process and
further research and investigation is needed in this area.

5METABOLIC ENGINEERING IN E.COLI
References
Janßen, H.J. and Steinbüchel, A., 2014. Fatty acid synthesis in Escherichia coli and its
applications towards the production of fatty acid based biofuels. Biotechnology for
biofuels, 7(1), p.7.
Jeon, E.Y., Lee, S.H., Lee, S.H., Han, S.O., Yoon, Y.J. and Lee, J.W., 2012. Improved
production of long-chain fatty acid in Escherichia coli by an engineering elongation cycle
during fatty acid synthesis (FAS) through genetic manipulation. Journal of microbiology and
biotechnology, 22(7), pp.990-999.
Lennen, R.M., Kruziki, M.A., Kumar, K., Zinkel, R.A., Burnum, K.E., Lipton, M.S., Hoover,
S.W., Ranatunga, D.R., Wittkopp, T.M., Marner, W.D. and Pfleger, B.F., 2011. Membrane
stresses induced by overproduction of free fatty acids in Escherichia coli. Applied and
environmental microbiology, 77(22), pp.8114-8128.
Liu, H., Yu, C., Feng, D., Cheng, T., Meng, X., Liu, W., Zou, H. and Xian, M., 2012.
Production of extracellular fatty acid using engineered Escherichia coli. Microbial cell
factories, 11(1), p.41.
Schweizer, E. and Hofmann, J., 2004. Microbial type I fatty acid synthases (FAS): major
players in a network of cellular FAS systems. Microbiology and molecular biology
reviews, 68(3), pp.501-517.
Tee, T.W., Chowdhury, A., Maranas, C.D. and Shanks, J.V., 2014. Systems metabolic
engineering design: fatty acid production as an emerging case study. Biotechnology and
bioengineering, 111(5), pp.849-857.
Youngquist, J.T., Lennen, R.M., Ranatunga, D.R., Bothfeld, W.H., II, W.D.M. and Pfleger,
B.F., 2012. Kinetic modeling of free fatty acid production in Escherichia coli based on
References
Janßen, H.J. and Steinbüchel, A., 2014. Fatty acid synthesis in Escherichia coli and its
applications towards the production of fatty acid based biofuels. Biotechnology for
biofuels, 7(1), p.7.
Jeon, E.Y., Lee, S.H., Lee, S.H., Han, S.O., Yoon, Y.J. and Lee, J.W., 2012. Improved
production of long-chain fatty acid in Escherichia coli by an engineering elongation cycle
during fatty acid synthesis (FAS) through genetic manipulation. Journal of microbiology and
biotechnology, 22(7), pp.990-999.
Lennen, R.M., Kruziki, M.A., Kumar, K., Zinkel, R.A., Burnum, K.E., Lipton, M.S., Hoover,
S.W., Ranatunga, D.R., Wittkopp, T.M., Marner, W.D. and Pfleger, B.F., 2011. Membrane
stresses induced by overproduction of free fatty acids in Escherichia coli. Applied and
environmental microbiology, 77(22), pp.8114-8128.
Liu, H., Yu, C., Feng, D., Cheng, T., Meng, X., Liu, W., Zou, H. and Xian, M., 2012.
Production of extracellular fatty acid using engineered Escherichia coli. Microbial cell
factories, 11(1), p.41.
Schweizer, E. and Hofmann, J., 2004. Microbial type I fatty acid synthases (FAS): major
players in a network of cellular FAS systems. Microbiology and molecular biology
reviews, 68(3), pp.501-517.
Tee, T.W., Chowdhury, A., Maranas, C.D. and Shanks, J.V., 2014. Systems metabolic
engineering design: fatty acid production as an emerging case study. Biotechnology and
bioengineering, 111(5), pp.849-857.
Youngquist, J.T., Lennen, R.M., Ranatunga, D.R., Bothfeld, W.H., II, W.D.M. and Pfleger,
B.F., 2012. Kinetic modeling of free fatty acid production in Escherichia coli based on
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6METABOLIC ENGINEERING IN E.COLI
continuous cultivation of a plasmid free strain. Biotechnology and Bioengineering, 109(6),
pp.1518-1527.
Zhang, F., Carothers, J.M. and Keasling, J.D., 2012. Design of a dynamic sensor-regulator
system for production of chemicals and fuels derived from fatty acids. Nature
biotechnology, 30(4), p.354.
continuous cultivation of a plasmid free strain. Biotechnology and Bioengineering, 109(6),
pp.1518-1527.
Zhang, F., Carothers, J.M. and Keasling, J.D., 2012. Design of a dynamic sensor-regulator
system for production of chemicals and fuels derived from fatty acids. Nature
biotechnology, 30(4), p.354.
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




