Research Proposal: Methylglyoxal, TRAP1, and Diabetic Neuropathy
VerifiedAdded on 2023/04/21
|10
|3361
|303
Project
AI Summary
This research proposal investigates the role of methylglyoxal in the development of diabetic neuropathy. The study aims to determine the impact of methylglyoxal on the activation of TRAP1 and Na(v)1.8 channels in animal models, specifically male wistar rats. The methodology involves injecting methylglyoxal and formaldehyde, measuring flinching rates, blood glucose levels, and the effects of methylglyoxal scavengers. Statistical analysis using SPSS 21.0 software, ANOVA, and student-t tests will be conducted to analyze the data. The research seeks to establish a causal relationship between methylglyoxal and diabetic neuropathy, potentially leading to the identification of drug targets to alleviate neuropathic pain. The proposal includes detailed background information, the hypothesis, experimental design, and expected outcomes, along with a list of relevant references.

Running head: RESEARCH PROPOSAL
Research proposal
Name of the Student
Name of the University
Author Note
Research proposal
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
1RESEARCH PROPOSAL
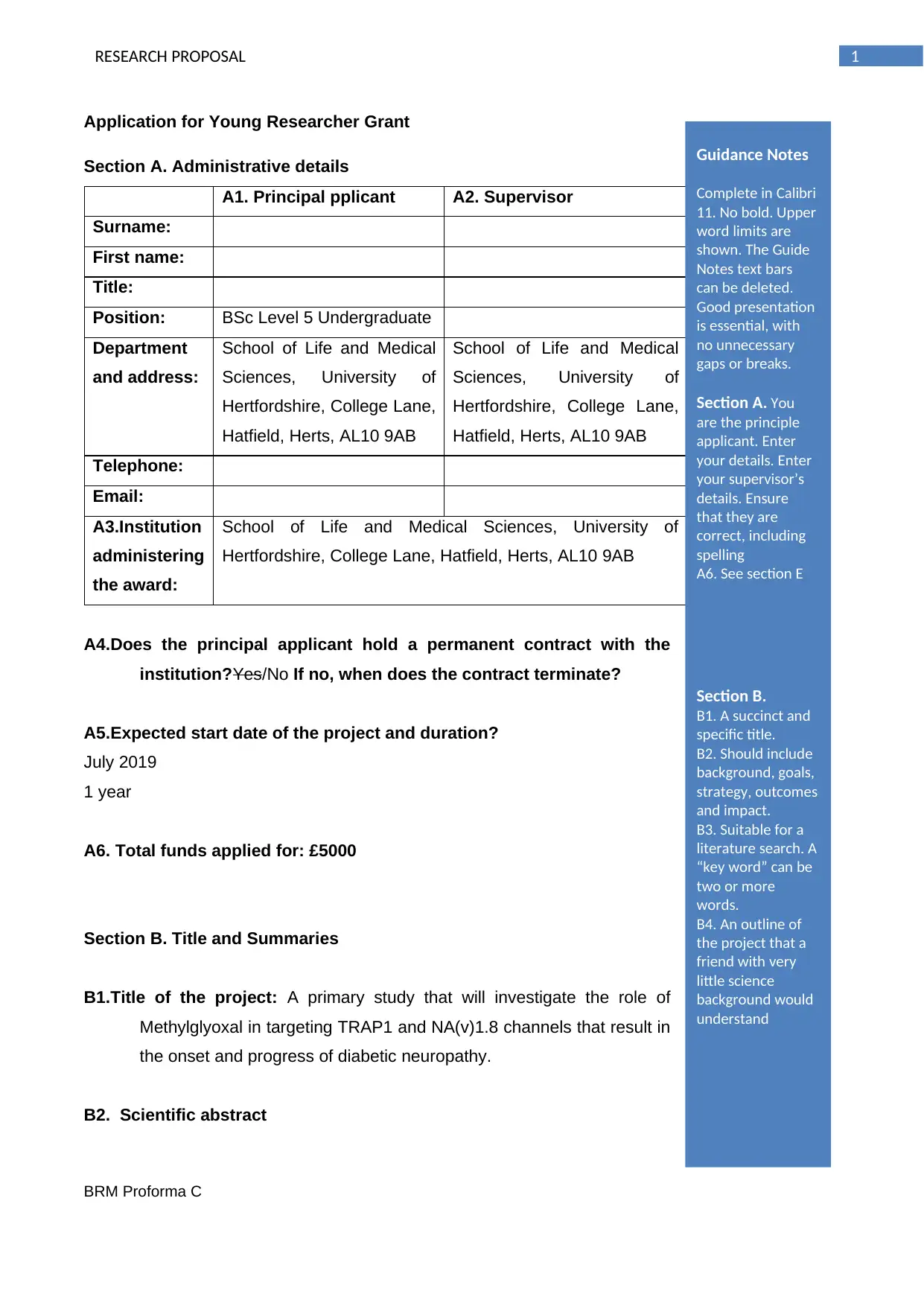
Application for Young Researcher Grant
Section A. Administrative details
A1. Principal pplicant A2. Supervisor
Surname:
First name:
Title:
Position: BSc Level 5 Undergraduate
Department
and address:
School of Life and Medical
Sciences, University of
Hertfordshire, College Lane,
Hatfield, Herts, AL10 9AB
School of Life and Medical
Sciences, University of
Hertfordshire, College Lane,
Hatfield, Herts, AL10 9AB
Telephone:
Email:
A3.Institution
administering
the award:
School of Life and Medical Sciences, University of
Hertfordshire, College Lane, Hatfield, Herts, AL10 9AB
A4.Does the principal applicant hold a permanent contract with the
institution?Yes/No If no, when does the contract terminate?
A5.Expected start date of the project and duration?
July 2019
1 year
A6. Total funds applied for: £5000
Section B. Title and Summaries
B1.Title of the project: A primary study that will investigate the role of
Methylglyoxal in targeting TRAP1 and NA(v)1.8 channels that result in
the onset and progress of diabetic neuropathy.
B2. Scientific abstract
BRM Proforma C
Guidance Notes
Complete in Calibri
11. No bold. Upper
word limits are
shown. The Guide
Notes text bars
can be deleted.
Good presentation
is essential, with
no unnecessary
gaps or breaks.
Section A. You
are the principle
applicant. Enter
your details. Enter
your supervisor’s
details. Ensure
that they are
correct, including
spelling
A6. See section E
Section B.
B1. A succinct and
specific title.
B2. Should include
background, goals,
strategy, outcomes
and impact.
B3. Suitable for a
literature search. A
“key word” can be
two or more
words.
B4. An outline of
the project that a
friend with very
little science
background would
understand
Application for Young Researcher Grant
Section A. Administrative details
A1. Principal pplicant A2. Supervisor
Surname:
First name:
Title:
Position: BSc Level 5 Undergraduate
Department
and address:
School of Life and Medical
Sciences, University of
Hertfordshire, College Lane,
Hatfield, Herts, AL10 9AB
School of Life and Medical
Sciences, University of
Hertfordshire, College Lane,
Hatfield, Herts, AL10 9AB
Telephone:
Email:
A3.Institution
administering
the award:
School of Life and Medical Sciences, University of
Hertfordshire, College Lane, Hatfield, Herts, AL10 9AB
A4.Does the principal applicant hold a permanent contract with the
institution?Yes/No If no, when does the contract terminate?
A5.Expected start date of the project and duration?
July 2019
1 year
A6. Total funds applied for: £5000
Section B. Title and Summaries
B1.Title of the project: A primary study that will investigate the role of
Methylglyoxal in targeting TRAP1 and NA(v)1.8 channels that result in
the onset and progress of diabetic neuropathy.
B2. Scientific abstract
BRM Proforma C
Guidance Notes
Complete in Calibri
11. No bold. Upper
word limits are
shown. The Guide
Notes text bars
can be deleted.
Good presentation
is essential, with
no unnecessary
gaps or breaks.
Section A. You
are the principle
applicant. Enter
your details. Enter
your supervisor’s
details. Ensure
that they are
correct, including
spelling
A6. See section E
Section B.
B1. A succinct and
specific title.
B2. Should include
background, goals,
strategy, outcomes
and impact.
B3. Suitable for a
literature search. A
“key word” can be
two or more
words.
B4. An outline of
the project that a
friend with very
little science
background would
understand

2RESEARCH PROPOSAL
Methylglyoxal is a dicarbonyl metabolite and typically forms under conditions of hyperglycemia. The
endogenous increase in the levels of methylglyoxal and AGE have also been allied with
development of several diabetes associated complications. This correlation can be significantly
related to onset of diabetic neuropathic pain owing to the vulnerability of peripheral neurons to
methylglyoxal accumulation, which in turn occurs due to a reduction in the levels of glyoxalase 1
enzyme. It is expected that endogenously upsurged levels of methylgloxal will be able to mediate
pain associated with diabetic neuropathy, by activating the TRAP1 and Na(v)1.8 channels that are
expressed on primary afferent sensory neurons. The research proposal aims to determine the
impact of methylglyoxal on development of diabetic neuropathic pain in animal models, by bringing
about an activation of TRAP1 and Na(v)1.8 channels. Male wistar rats will be taken and randomised
into experimental and control groups with methylglyoxal and formaldehyde injection in the hindpaw,
respectively. Their flinching rate will be measured in the observation chamber, in addition to
measurement of blood glucose levels, and effect of methylglyoxal scavengers. Descriptive statistical
analysis conducted with the help of SPSS 21.0 software, ANOVA and student-test test will help in
numerically representing the effects that methylglyoxal administration has brought about in the
winstar rats.
B3. Five key words relating to the proposal
Methylglyoxal, TRAP1, NA(v)1.8, diabetic neuropathy, biomarker
B4. Summary of the project in lay terms
Diabetic neuropathy is an umbrella term that comprises of several nerve damaging illness
that are related with diabetic mellitus. The conditions are typically found to occur due to diabetic
microvascular injuries that encompass minute blood vessels supplying to the nerves (vasa
nervorum), besides macrovascular conditions that lead to the onset of diabetic neuropathy (Rabbani
and Thornalley 2014). Methylglyoxal refers to an organic compound that is typically used in the form
of a reagent for different types of organic synthesis and are also utilised for tanning procedures.
This compound is extremely reactive and derived from fructose and glucose metabolism (Allaman,
Bélanger and Magistretti 2015). Owing to the fact that it has been implicated to play an important
role in different diabetes associated complications such as, psychiatric comorbidity, retinopathy, and
nephropathy, there is a need to determine the impacts it exerts on neuropathic pain.
This research will determine the effect of methylglyoxal on the development of diabetic neuropathy.
The proposed research will try to explore the association between methylglyoxal and development
of diabetic neuropathic pain, via the activation of TRAP1 and Na(v)1.8 channels. It will involve
alloxane and methylglyoxal-induced rat pain models that will be assessed for investigating the
BRM Proforma C
Methylglyoxal is a dicarbonyl metabolite and typically forms under conditions of hyperglycemia. The
endogenous increase in the levels of methylglyoxal and AGE have also been allied with
development of several diabetes associated complications. This correlation can be significantly
related to onset of diabetic neuropathic pain owing to the vulnerability of peripheral neurons to
methylglyoxal accumulation, which in turn occurs due to a reduction in the levels of glyoxalase 1
enzyme. It is expected that endogenously upsurged levels of methylgloxal will be able to mediate
pain associated with diabetic neuropathy, by activating the TRAP1 and Na(v)1.8 channels that are
expressed on primary afferent sensory neurons. The research proposal aims to determine the
impact of methylglyoxal on development of diabetic neuropathic pain in animal models, by bringing
about an activation of TRAP1 and Na(v)1.8 channels. Male wistar rats will be taken and randomised
into experimental and control groups with methylglyoxal and formaldehyde injection in the hindpaw,
respectively. Their flinching rate will be measured in the observation chamber, in addition to
measurement of blood glucose levels, and effect of methylglyoxal scavengers. Descriptive statistical
analysis conducted with the help of SPSS 21.0 software, ANOVA and student-test test will help in
numerically representing the effects that methylglyoxal administration has brought about in the
winstar rats.
B3. Five key words relating to the proposal
Methylglyoxal, TRAP1, NA(v)1.8, diabetic neuropathy, biomarker
B4. Summary of the project in lay terms
Diabetic neuropathy is an umbrella term that comprises of several nerve damaging illness
that are related with diabetic mellitus. The conditions are typically found to occur due to diabetic
microvascular injuries that encompass minute blood vessels supplying to the nerves (vasa
nervorum), besides macrovascular conditions that lead to the onset of diabetic neuropathy (Rabbani
and Thornalley 2014). Methylglyoxal refers to an organic compound that is typically used in the form
of a reagent for different types of organic synthesis and are also utilised for tanning procedures.
This compound is extremely reactive and derived from fructose and glucose metabolism (Allaman,
Bélanger and Magistretti 2015). Owing to the fact that it has been implicated to play an important
role in different diabetes associated complications such as, psychiatric comorbidity, retinopathy, and
nephropathy, there is a need to determine the impacts it exerts on neuropathic pain.
This research will determine the effect of methylglyoxal on the development of diabetic neuropathy.
The proposed research will try to explore the association between methylglyoxal and development
of diabetic neuropathic pain, via the activation of TRAP1 and Na(v)1.8 channels. It will involve
alloxane and methylglyoxal-induced rat pain models that will be assessed for investigating the
BRM Proforma C
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3RESEARCH PROPOSAL
effects on Na(v)1.8 antagonist and TRAP1 antagonist. Co-incubation with methylglyoxal scavengers
will also help in ascertaining the role that the metabolite exerts on the receptor channels. The
research would require 8 male wistar rats and the records will be noted over a period of four weeks.
Section C. Description of the project
C1. Background to the project
Methylglyoxal has been recognised as a physiological dicarbonyl
metabolite that is primarily produced from triosephosphate glycolysis, and gets
detoxified by two different enzymes namely, glyoxalase 2 and glyoxalase 1, to
form the end product D-lactate (Allaman, Bélanger and Magistretti 2015).
Diabetic associated health complications are major problems that have been
recognised on a worldwide basis, with the cost for treatment of diabetes
increasing to as much as US$245 billion in the year 2012 (Beisswenger 2014).
Several research evidences have elaborated on the fact that detoxification and
production of methylglyoxal is controlled by a range of enzymatic mechanisms,
environmental factors and genetic factors that govern tissue glycation and
account for the rates of complication (Rabbani and Thornalley 2014). In
addition, evidences have also suggested that development and accumulation
of advanced glycation end-products (AGE) result in the progress of diabetes
and associated comorbidities (Maessen, Stehouwer and Schalkwijk 2015).
Methylglyoxal acts as a precursor for AGE formation, and an endogenous
increase in its level has been associated with the manifestation of retinopathy,
nephropathy, and other diabetes complications. Further correlation can also be
associated to the onset of diabetic neuropathic pain, owing to the vulnerability
of peripheral neurones to methylglyoxal accumulation, due to a lessening in
expression of glyoxalase 1.
C2. Aim of the project
In this project the primary aim is to investigate the association between
increased levels of methylglyoxal and development of neuropathic pain in
animal models, through activation of TRAP1 and Na(v)1.8 channels.
C3. Scientific objective and criteria for success
BRM Proforma C
Section C. C1. A
brief description of
basicfacts, current
theory,recent
evidence, the
question addressed
and the strategy.
C2. A goal defined
by the hypothesis.
Note it may not be
achieved even if the
objective has been
achieved.
C3. A goal defined
by completion of the
experimental work.
An example of
success criteria may
be size and quality
of the data.
C4. The supposed
explanation stated in
concise and precise
terms. The null is the
actual hypothesis
that you attempt to
disprove in the
experiments.
C5. Outline strategy
and methodology,
with only enough
detail to make clear
how the objective
will be achieved.
C6. Will outcomes
matter in applied or
fundamental,
narrow or broad
fields of science, and
impact on the
theory studied and
on major theories?
Will society be
influenced,
eg.human or animal
welfare, business,
technology, policy
etc.?
C7. Four recent
original research
papers listed in full
in the correct
format.
effects on Na(v)1.8 antagonist and TRAP1 antagonist. Co-incubation with methylglyoxal scavengers
will also help in ascertaining the role that the metabolite exerts on the receptor channels. The
research would require 8 male wistar rats and the records will be noted over a period of four weeks.
Section C. Description of the project
C1. Background to the project
Methylglyoxal has been recognised as a physiological dicarbonyl
metabolite that is primarily produced from triosephosphate glycolysis, and gets
detoxified by two different enzymes namely, glyoxalase 2 and glyoxalase 1, to
form the end product D-lactate (Allaman, Bélanger and Magistretti 2015).
Diabetic associated health complications are major problems that have been
recognised on a worldwide basis, with the cost for treatment of diabetes
increasing to as much as US$245 billion in the year 2012 (Beisswenger 2014).
Several research evidences have elaborated on the fact that detoxification and
production of methylglyoxal is controlled by a range of enzymatic mechanisms,
environmental factors and genetic factors that govern tissue glycation and
account for the rates of complication (Rabbani and Thornalley 2014). In
addition, evidences have also suggested that development and accumulation
of advanced glycation end-products (AGE) result in the progress of diabetes
and associated comorbidities (Maessen, Stehouwer and Schalkwijk 2015).
Methylglyoxal acts as a precursor for AGE formation, and an endogenous
increase in its level has been associated with the manifestation of retinopathy,
nephropathy, and other diabetes complications. Further correlation can also be
associated to the onset of diabetic neuropathic pain, owing to the vulnerability
of peripheral neurones to methylglyoxal accumulation, due to a lessening in
expression of glyoxalase 1.
C2. Aim of the project
In this project the primary aim is to investigate the association between
increased levels of methylglyoxal and development of neuropathic pain in
animal models, through activation of TRAP1 and Na(v)1.8 channels.
C3. Scientific objective and criteria for success
BRM Proforma C
Section C. C1. A
brief description of
basicfacts, current
theory,recent
evidence, the
question addressed
and the strategy.
C2. A goal defined
by the hypothesis.
Note it may not be
achieved even if the
objective has been
achieved.
C3. A goal defined
by completion of the
experimental work.
An example of
success criteria may
be size and quality
of the data.
C4. The supposed
explanation stated in
concise and precise
terms. The null is the
actual hypothesis
that you attempt to
disprove in the
experiments.
C5. Outline strategy
and methodology,
with only enough
detail to make clear
how the objective
will be achieved.
C6. Will outcomes
matter in applied or
fundamental,
narrow or broad
fields of science, and
impact on the
theory studied and
on major theories?
Will society be
influenced,
eg.human or animal
welfare, business,
technology, policy
etc.?
C7. Four recent
original research
papers listed in full
in the correct
format.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4RESEARCH PROPOSAL
The major objective is to examine the association between levels of plasma methylglyoxal
and TRAP1 and Na(v)1.8 channel activation, which lead to diabetic neuropathy. Determining the
association between diabetic neuropathy and this dicarbonate metabolite will help in the
identification and administration of peptide inhibitors that will treat hypersensitivity to pain among
diabetic patients.
C4. Main hypothesis addressed and null hypothesis to be tested
H1- Methylglyoxal will mediate alloxan-induced diabetic neuropathy through the activation of
peripheral Na(v)1.8 and TRAP1channels.
H0- Methylglyoxal will not exert any effect on alloxan-induced diabetic neuropathy through
the activation of peripheral Na(v)1.8 and TRAP1channels.
C5. Outline plan of experimental work including statistical analysis
Following the purchase of 40% methylglyoxal and 37-40% formaldehyde solution,
A803467, commonly referred to as the blocker of Na(v)1.8 sodium channel, and A967079, the
selective antagonist for TRAP1 receptors will be obtained. Other necessary chemicals required for
the project apart from alloxan and metformin hydrochloride will also be purchased. The research will
involve randomisation of male wistar rats to the experimental (Methylglyoxal) and control group
(formaldehyde) in equal proportion. Methylglyoxal and/or formaldehyde will be subcutaneously
injected in the dorsal side of left hindpaw, following which the rats will be placed in the observation
chambers. Recording the incidence of flinching for a 1 minute period, for every 10 minutes (for
formaldehyde) and five minutes (for methylglyoxal), over an hour, will help in quantification of their
behaviour.
After studying the rats for 20 hours, prior to intravenous injection of alloxan, a behavioral
assessment will be conducted. The rats will be made to stand on a metal grid, following which the
threshold of hindpaw withdrawal, evoked via hindpaw stimulation by an Electronic von Frey will be
assessed. The threshold will be associated with the lowest force of a monofilament that will
successfully bring about a withdrawal response in the rats. Blood sugar levels will be measured on
a weekly basis for four weeks, following subcutaneous injection of alloxane. Blood collection from
orbit flexus will be conducted when the rats are under mild anaesthetic conditions, at the similar
time frame, and the plasma will be frozen until measurements for methylglyoxal has been done. Co-
incubation will be conducted with methylglyoxal scavengers, D-arginine, metformin, and
aminoguadine, to assess the levels of free methylgloycal present, in relation to nociception. A dose
dependent curve analysis will be conducted for determining the statistical relations between the
variables. Two-way repeated measures analysis of variance (ANOVA) and student-T test will be
BRM Proforma C
The major objective is to examine the association between levels of plasma methylglyoxal
and TRAP1 and Na(v)1.8 channel activation, which lead to diabetic neuropathy. Determining the
association between diabetic neuropathy and this dicarbonate metabolite will help in the
identification and administration of peptide inhibitors that will treat hypersensitivity to pain among
diabetic patients.
C4. Main hypothesis addressed and null hypothesis to be tested
H1- Methylglyoxal will mediate alloxan-induced diabetic neuropathy through the activation of
peripheral Na(v)1.8 and TRAP1channels.
H0- Methylglyoxal will not exert any effect on alloxan-induced diabetic neuropathy through
the activation of peripheral Na(v)1.8 and TRAP1channels.
C5. Outline plan of experimental work including statistical analysis
Following the purchase of 40% methylglyoxal and 37-40% formaldehyde solution,
A803467, commonly referred to as the blocker of Na(v)1.8 sodium channel, and A967079, the
selective antagonist for TRAP1 receptors will be obtained. Other necessary chemicals required for
the project apart from alloxan and metformin hydrochloride will also be purchased. The research will
involve randomisation of male wistar rats to the experimental (Methylglyoxal) and control group
(formaldehyde) in equal proportion. Methylglyoxal and/or formaldehyde will be subcutaneously
injected in the dorsal side of left hindpaw, following which the rats will be placed in the observation
chambers. Recording the incidence of flinching for a 1 minute period, for every 10 minutes (for
formaldehyde) and five minutes (for methylglyoxal), over an hour, will help in quantification of their
behaviour.
After studying the rats for 20 hours, prior to intravenous injection of alloxan, a behavioral
assessment will be conducted. The rats will be made to stand on a metal grid, following which the
threshold of hindpaw withdrawal, evoked via hindpaw stimulation by an Electronic von Frey will be
assessed. The threshold will be associated with the lowest force of a monofilament that will
successfully bring about a withdrawal response in the rats. Blood sugar levels will be measured on
a weekly basis for four weeks, following subcutaneous injection of alloxane. Blood collection from
orbit flexus will be conducted when the rats are under mild anaesthetic conditions, at the similar
time frame, and the plasma will be frozen until measurements for methylglyoxal has been done. Co-
incubation will be conducted with methylglyoxal scavengers, D-arginine, metformin, and
aminoguadine, to assess the levels of free methylgloycal present, in relation to nociception. A dose
dependent curve analysis will be conducted for determining the statistical relations between the
variables. Two-way repeated measures analysis of variance (ANOVA) and student-T test will be
BRM Proforma C

5RESEARCH PROPOSAL
conducted to determine statistical significance. The SPSS 21.0 software will
be used for the purpose.
C6. Significance and impact of expected outcomes
Although several studies have revealed the association between
increased levels of plasma methylglyoxal and diabetes, there is little evidence
regarding the potential association between methylglyoxal with TRAP1 and
Na(v)1.8 channel activation, which is responsible for the onset of diabetic
neuropathy. Establishing causal relationship between the two with the help of
animal models will facilitate identification of potential pathways that can be
targeted by drugs for reducing the security of diabetic neuropathic pain.
Hence, the research findings will have significance in drug target recognition.
C7. References (provide 4)
Allaman, I., Bélanger, M. and Magistretti, P.J., 2015. Methylglyoxal, the dark
side of glycolysis. Frontiers in neuroscience, 9, p.23.
Beisswenger, P.J., 2014. Methylglyoxal in diabetes: link to treatment,
glycaemic control and biomarkers of complications. Biochemical Society
Transactions, 42(2), pp.450-456.
Maessen, D.E., Stehouwer, C.D. and Schalkwijk, C.G., 2015. The role of
methylglyoxal and the glyoxalase system in diabetes and other age-related
diseases. Clinical science, 128(12), pp.839-861.
Rabbani, N. and Thornalley, P.J., 2014. The critical role of methylglyoxal and
glyoxalase 1 in diabetic nephropathy. Diabetes, 63(1), pp.50-52.
Section D. Experiments on humans or other animals
(answer all questions)
D1. Will the project involve human subjects? Yes/no
D2. What are the primary concerns regarding the use of human
subjects? (35 words)
BRM Proforma C
D2. If D1 is yes,
answer specifically,
if no, then
generally. eg.
health risks, levels
of distress (physical
psychological)shoul
d be zero, or
minimal, give a
generic example,
with nature of risk
and safeguards.
D3. For example,
will subjects be
anonymous, and
data restricted
access and time
limited?
D5. If D4 is yes,
answer specifically,
if no, then
generally, with
mouse as an
example. Provide
the Latin name in
the proper format.
How many animals
will be used at
most?
D6. Are animals
essential to this
study? If not, then
in any study?
D7. Might include
comments on
facilities, licences
held and steps to be
taken to pursue
replacement
reduction and
refinement.
E1. One example for
each category.
Provide cost per
unit, number of
units required and
total cost. Units
here are purchase
units, in weight or
volume, or activity
units, numbers etc.
Plasticware include
pipette tips, micro-
centrifuge tubes
conducted to determine statistical significance. The SPSS 21.0 software will
be used for the purpose.
C6. Significance and impact of expected outcomes
Although several studies have revealed the association between
increased levels of plasma methylglyoxal and diabetes, there is little evidence
regarding the potential association between methylglyoxal with TRAP1 and
Na(v)1.8 channel activation, which is responsible for the onset of diabetic
neuropathy. Establishing causal relationship between the two with the help of
animal models will facilitate identification of potential pathways that can be
targeted by drugs for reducing the security of diabetic neuropathic pain.
Hence, the research findings will have significance in drug target recognition.
C7. References (provide 4)
Allaman, I., Bélanger, M. and Magistretti, P.J., 2015. Methylglyoxal, the dark
side of glycolysis. Frontiers in neuroscience, 9, p.23.
Beisswenger, P.J., 2014. Methylglyoxal in diabetes: link to treatment,
glycaemic control and biomarkers of complications. Biochemical Society
Transactions, 42(2), pp.450-456.
Maessen, D.E., Stehouwer, C.D. and Schalkwijk, C.G., 2015. The role of
methylglyoxal and the glyoxalase system in diabetes and other age-related
diseases. Clinical science, 128(12), pp.839-861.
Rabbani, N. and Thornalley, P.J., 2014. The critical role of methylglyoxal and
glyoxalase 1 in diabetic nephropathy. Diabetes, 63(1), pp.50-52.
Section D. Experiments on humans or other animals
(answer all questions)
D1. Will the project involve human subjects? Yes/no
D2. What are the primary concerns regarding the use of human
subjects? (35 words)
BRM Proforma C
D2. If D1 is yes,
answer specifically,
if no, then
generally. eg.
health risks, levels
of distress (physical
psychological)shoul
d be zero, or
minimal, give a
generic example,
with nature of risk
and safeguards.
D3. For example,
will subjects be
anonymous, and
data restricted
access and time
limited?
D5. If D4 is yes,
answer specifically,
if no, then
generally, with
mouse as an
example. Provide
the Latin name in
the proper format.
How many animals
will be used at
most?
D6. Are animals
essential to this
study? If not, then
in any study?
D7. Might include
comments on
facilities, licences
held and steps to be
taken to pursue
replacement
reduction and
refinement.
E1. One example for
each category.
Provide cost per
unit, number of
units required and
total cost. Units
here are purchase
units, in weight or
volume, or activity
units, numbers etc.
Plasticware include
pipette tips, micro-
centrifuge tubes
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6RESEARCH PROPOSAL
The most basic concerns that are related to the use of human participants for research are
beneficence, non‐maleficence, personal dignity, trust, and autonomy related to voluntary consent for
participation in the research. Confidentiality of the participants must also be maintained.
D3. How is provision of data protection for human subjects ensured?(35 words)
The provision for data protection for human research participants is ensured by the Data
Protection Act 1998 that safeguards confidentiality of all participants. It also considers sensitivirt of
the information that has been gathered during research, and imposes responsibilities on
researchers use and record participant information.
D4. Will live animals will be involved in the study?Yes/no
D5. Identify the species and provide an indication of numbers and life stage.
Male wistar rats are an albino outbred of Rattus norvegicus domesticus species. 8 rats, each
aged three weeks and above will be taken for the research. They will be equally divided in the
experimental and control group (four each).
D6. What is the rationale for their use?
These rats will be used for research because their biological behaviour and genetic
characteristics closely resemble humans, and human metabolic conditions can be easily replicated
in them.
D7. What provision will/can be made to ensure animal welfare and reduce animal usage with
reference to the 3 Rs?
The principles of 3Rs focus on replacement, reduction, and refinement. The investigation
would encompass fewer animals and would refine the experiments in a manner that the animals do
not face any major suffering. The observation chamber would have a feasible environment, which
would minimise pain and suffering of the rats, with the aim of improving animal welfare. The number
of rats required for the research is also minimal, for an experimental study.
Section E. Budget breakdown
BRM Proforma C
The most basic concerns that are related to the use of human participants for research are
beneficence, non‐maleficence, personal dignity, trust, and autonomy related to voluntary consent for
participation in the research. Confidentiality of the participants must also be maintained.
D3. How is provision of data protection for human subjects ensured?(35 words)
The provision for data protection for human research participants is ensured by the Data
Protection Act 1998 that safeguards confidentiality of all participants. It also considers sensitivirt of
the information that has been gathered during research, and imposes responsibilities on
researchers use and record participant information.
D4. Will live animals will be involved in the study?Yes/no
D5. Identify the species and provide an indication of numbers and life stage.
Male wistar rats are an albino outbred of Rattus norvegicus domesticus species. 8 rats, each
aged three weeks and above will be taken for the research. They will be equally divided in the
experimental and control group (four each).
D6. What is the rationale for their use?
These rats will be used for research because their biological behaviour and genetic
characteristics closely resemble humans, and human metabolic conditions can be easily replicated
in them.
D7. What provision will/can be made to ensure animal welfare and reduce animal usage with
reference to the 3 Rs?
The principles of 3Rs focus on replacement, reduction, and refinement. The investigation
would encompass fewer animals and would refine the experiments in a manner that the animals do
not face any major suffering. The observation chamber would have a feasible environment, which
would minimise pain and suffering of the rats, with the aim of improving animal welfare. The number
of rats required for the research is also minimal, for an experimental study.
Section E. Budget breakdown
BRM Proforma C
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
7RESEARCH PROPOSAL
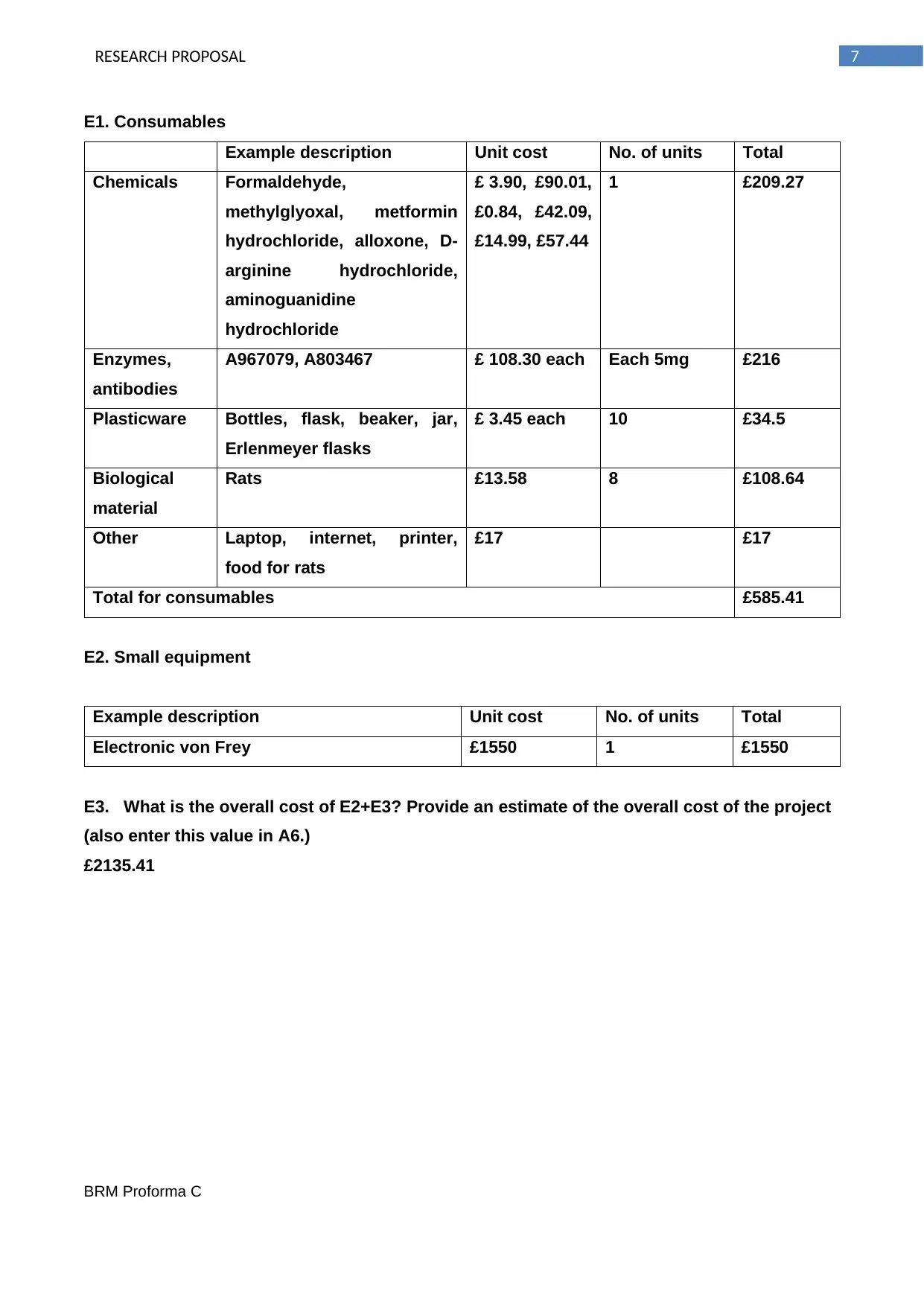
E1. Consumables
Example description Unit cost No. of units Total
Chemicals Formaldehyde,
methylglyoxal, metformin
hydrochloride, alloxone, D-
arginine hydrochloride,
aminoguanidine
hydrochloride
£ 3.90, £90.01,
£0.84, £42.09,
£14.99, £57.44
1 £209.27
Enzymes,
antibodies
A967079, A803467 £ 108.30 each Each 5mg £216
Plasticware Bottles, flask, beaker, jar,
Erlenmeyer flasks
£ 3.45 each 10 £34.5
Biological
material
Rats £13.58 8 £108.64
Other Laptop, internet, printer,
food for rats
£17 £17
Total for consumables £585.41
E2. Small equipment
Example description Unit cost No. of units Total
Electronic von Frey £1550 1 £1550
E3. What is the overall cost of E2+E3? Provide an estimate of the overall cost of the project
(also enter this value in A6.)
£2135.41
BRM Proforma C
E1. Consumables
Example description Unit cost No. of units Total
Chemicals Formaldehyde,
methylglyoxal, metformin
hydrochloride, alloxone, D-
arginine hydrochloride,
aminoguanidine
hydrochloride
£ 3.90, £90.01,
£0.84, £42.09,
£14.99, £57.44
1 £209.27
Enzymes,
antibodies
A967079, A803467 £ 108.30 each Each 5mg £216
Plasticware Bottles, flask, beaker, jar,
Erlenmeyer flasks
£ 3.45 each 10 £34.5
Biological
material
Rats £13.58 8 £108.64
Other Laptop, internet, printer,
food for rats
£17 £17
Total for consumables £585.41
E2. Small equipment
Example description Unit cost No. of units Total
Electronic von Frey £1550 1 £1550
E3. What is the overall cost of E2+E3? Provide an estimate of the overall cost of the project
(also enter this value in A6.)
£2135.41
BRM Proforma C

8RESEARCH PROPOSAL
Section F. Health and Safety
F1. Explain the Health and Safety procedures, documents and approvals
that will need to be completed prior to starting work.
Prior permission will be required from the Animal Care and Welfare
Committee of the university. The UK regulations on conducting any research
using animals will be strictly adhered to, prior to implementing any procedure
on the rats. Project licence will also be taken for carrying out the program of
research. The research will also be conducted in accordance to the Health and
Safety at Work etc. Act 1974
F2.What aspect of the work is most likely to present a hazard?
Physical hazards can include bites, cuts or scratches from the animals.
In addition, formaldehyde to be used in the control group is extremely
flammable, and might result in an explosion or upon inhalation it can lead to
cough, headache, shortness of breath, and burning sensation.
F3. What aspect of the work is most likely to increase risk?
Of the several risks that can be encountered while conducting this
research, there is an increase the likelihood of zoonoses to occur. This type of
disease gets naturally transmitted between humans and animals. The
condition can arise, if the animals have not been acquired healthy from
commercial sources, and are not kept in a protective environment.
Hypersensitivity reactions to particular physical substance or chemicals, like
allergen are another source of potential risk. All efforts must be taken to
prevent physical hazards that might occur due to economic injuries, apart from
animal bites and scratches.
F4. Under what circumstances might ethical approval needed?
The UK has some strict regulations related to animal research, and
makes it illegal to use and animal under circumstances, where alternative
known animal procedures are available. Ethical approval will be required from
the government, in relation to three licenses namely, (i) personal license for
the person carrying out the experiment, (ii) project license for all the
procedures, and (iii) establishment license for the department undertaking the
research project.
BRM Proforma C
F1. Answer in
general, but giving
one or two specific
examples
F2. For example
identify one or
more hazardous
chemicals or
procedures and
explain the hazard
F3. All procedures
should counter
hazards and
reduce risk, but
where is the
greatest concern?
F4. Answer in
general, but give
specific
information
related to the
proposal if
appropriate
Section F. Health and Safety
F1. Explain the Health and Safety procedures, documents and approvals
that will need to be completed prior to starting work.
Prior permission will be required from the Animal Care and Welfare
Committee of the university. The UK regulations on conducting any research
using animals will be strictly adhered to, prior to implementing any procedure
on the rats. Project licence will also be taken for carrying out the program of
research. The research will also be conducted in accordance to the Health and
Safety at Work etc. Act 1974
F2.What aspect of the work is most likely to present a hazard?
Physical hazards can include bites, cuts or scratches from the animals.
In addition, formaldehyde to be used in the control group is extremely
flammable, and might result in an explosion or upon inhalation it can lead to
cough, headache, shortness of breath, and burning sensation.
F3. What aspect of the work is most likely to increase risk?
Of the several risks that can be encountered while conducting this
research, there is an increase the likelihood of zoonoses to occur. This type of
disease gets naturally transmitted between humans and animals. The
condition can arise, if the animals have not been acquired healthy from
commercial sources, and are not kept in a protective environment.
Hypersensitivity reactions to particular physical substance or chemicals, like
allergen are another source of potential risk. All efforts must be taken to
prevent physical hazards that might occur due to economic injuries, apart from
animal bites and scratches.
F4. Under what circumstances might ethical approval needed?
The UK has some strict regulations related to animal research, and
makes it illegal to use and animal under circumstances, where alternative
known animal procedures are available. Ethical approval will be required from
the government, in relation to three licenses namely, (i) personal license for
the person carrying out the experiment, (ii) project license for all the
procedures, and (iii) establishment license for the department undertaking the
research project.
BRM Proforma C
F1. Answer in
general, but giving
one or two specific
examples
F2. For example
identify one or
more hazardous
chemicals or
procedures and
explain the hazard
F3. All procedures
should counter
hazards and
reduce risk, but
where is the
greatest concern?
F4. Answer in
general, but give
specific
information
related to the
proposal if
appropriate
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
9RESEARCH PROPOSAL
BRM Proforma C
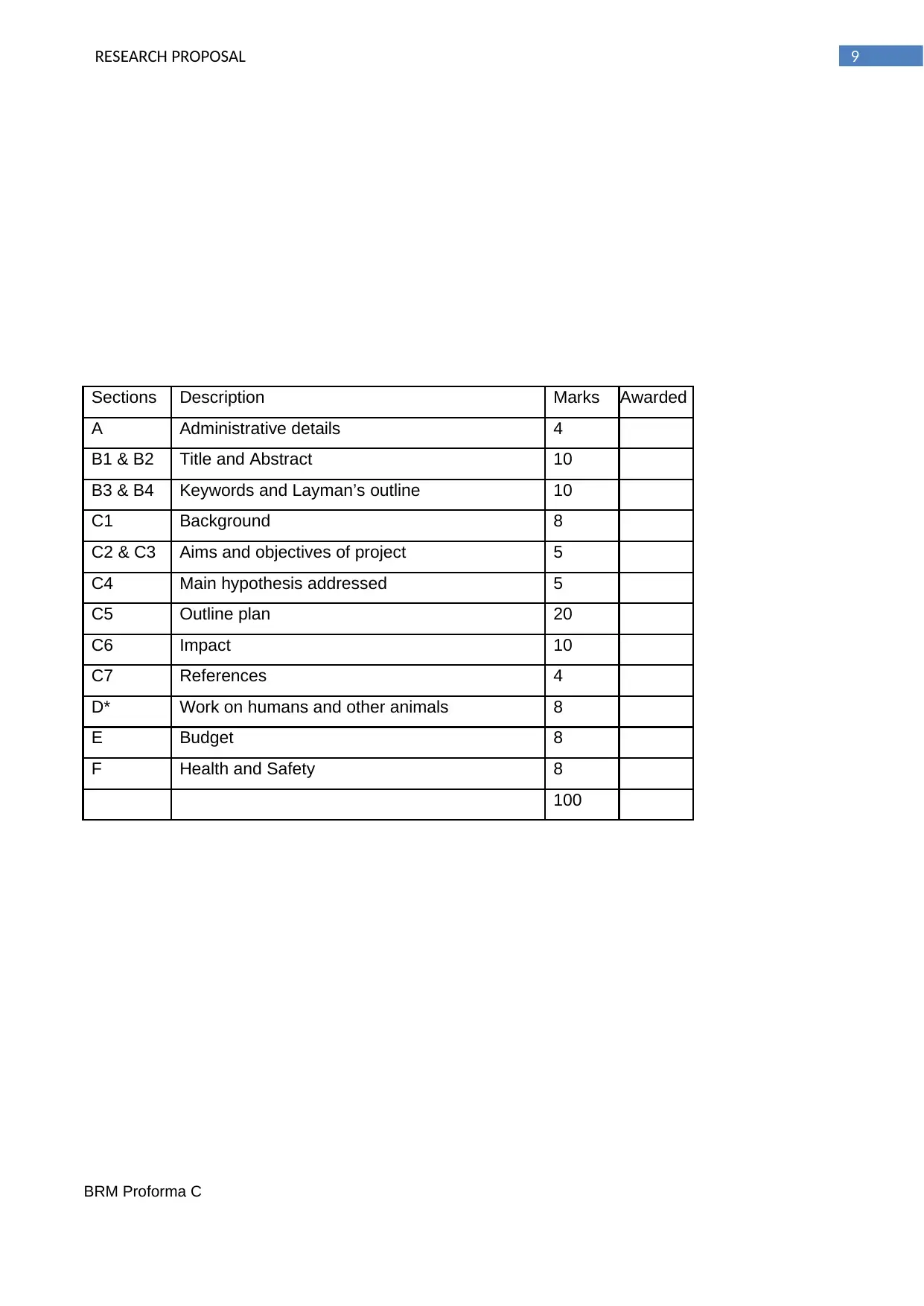
Sections Description Marks Awarded
A Administrative details 4
B1 & B2 Title and Abstract 10
B3 & B4 Keywords and Layman’s outline 10
C1 Background 8
C2 & C3 Aims and objectives of project 5
C4 Main hypothesis addressed 5
C5 Outline plan 20
C6 Impact 10
C7 References 4
D* Work on humans and other animals 8
E Budget 8
F Health and Safety 8
100
BRM Proforma C
Sections Description Marks Awarded
A Administrative details 4
B1 & B2 Title and Abstract 10
B3 & B4 Keywords and Layman’s outline 10
C1 Background 8
C2 & C3 Aims and objectives of project 5
C4 Main hypothesis addressed 5
C5 Outline plan 20
C6 Impact 10
C7 References 4
D* Work on humans and other animals 8
E Budget 8
F Health and Safety 8
100
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.