Analysis of the Carburizing Process on Mild Steel Properties
VerifiedAdded on 2022/08/11
|9
|1650
|18
Report
AI Summary
This report details the carburizing process applied to mild steel to enhance its mechanical properties. The study investigates the effects of heat treatment, including hardening, tempering, and case hardening, with a specific focus on carburizing. The methodology involves using cylindrical mild steel rods subjected to carburizing materials (barium carbonate and charcoal) and water quenching. The report presents results from hardness, tensile, and micro-structure evaluations, comparing water-quenched and case-hardened samples. Findings indicate that water quenching results in a martensitic microstructure with high hardness but increased brittleness, while case hardening increases surface hardness. The report concludes that the carburization process and the quenching medium significantly influence the mechanical properties of mild steel, affecting its application in various engineering contexts. Detailed chemical analysis and tensile strength tests are also included, providing a comprehensive analysis of the carburizing impact on mild steel.

1
Process cycle of Mild Steel
Name
Institutional Affiliation
Process cycle of Mild Steel
Name
Institutional Affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
Process Cycle of Mild Steel
Introduction
Heat treatment is a process of exposing metal in solid state to more
temperature cycles to obtain desired characteristics. The common type of heat
treatment includes hardening, tempering, annealing, and case hardening, us-
tempering and normalizing. Hardening process induces hardness property
(materialistic phase) to the metal rod. Tempering process soften and improves the
ductility of the metal stress relief reducing the brittleness and making the mild
steel tough to resist fatigue and shock (Fadara D. A., 2011). Case hardening
hardens the surface of the metal and not the core section part. The case hardening
types includes nitriding, carburizing, cyaniding and carbon-nitriding. Reactivity
and nature of carbon affects the mechanical properties and resistance of the mild
steel. Failure of the mild steel in the applications in bridges, building
reinforcement causes collapsing. The report concentrates on carburizing which is
a form of case hardening which involves adding carbon to the surface of mild
carbon.
Objectives
The main aim for the study were;
i. To determine the impacts of heat treatment to mechanical
properties of mild steel.
ii. To determine the most appropriate quenchant.
Material requirements
Process Cycle of Mild Steel
Introduction
Heat treatment is a process of exposing metal in solid state to more
temperature cycles to obtain desired characteristics. The common type of heat
treatment includes hardening, tempering, annealing, and case hardening, us-
tempering and normalizing. Hardening process induces hardness property
(materialistic phase) to the metal rod. Tempering process soften and improves the
ductility of the metal stress relief reducing the brittleness and making the mild
steel tough to resist fatigue and shock (Fadara D. A., 2011). Case hardening
hardens the surface of the metal and not the core section part. The case hardening
types includes nitriding, carburizing, cyaniding and carbon-nitriding. Reactivity
and nature of carbon affects the mechanical properties and resistance of the mild
steel. Failure of the mild steel in the applications in bridges, building
reinforcement causes collapsing. The report concentrates on carburizing which is
a form of case hardening which involves adding carbon to the surface of mild
carbon.
Objectives
The main aim for the study were;
i. To determine the impacts of heat treatment to mechanical
properties of mild steel.
ii. To determine the most appropriate quenchant.
Material requirements
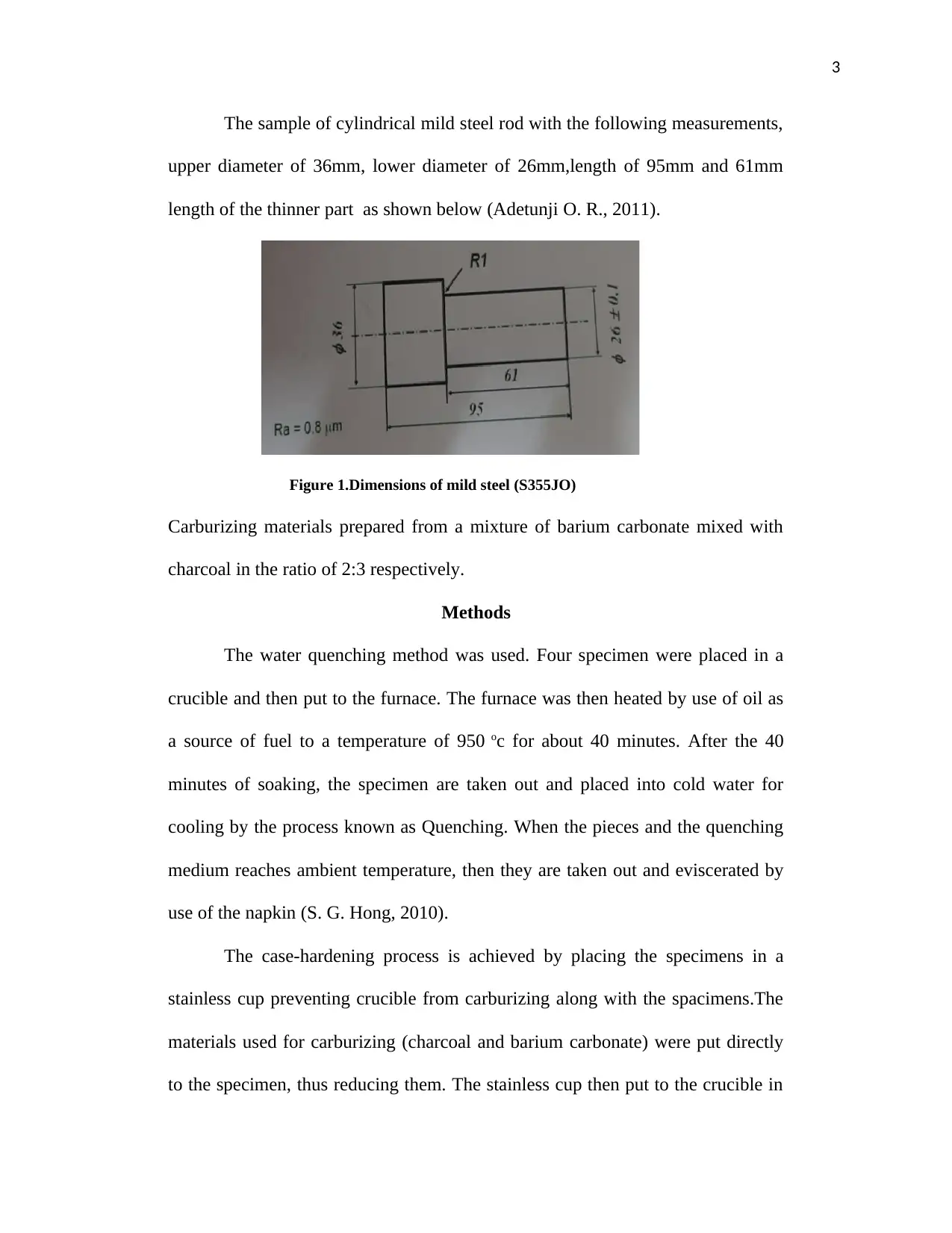
3
The sample of cylindrical mild steel rod with the following measurements,
upper diameter of 36mm, lower diameter of 26mm,length of 95mm and 61mm
length of the thinner part as shown below (Adetunji O. R., 2011).
Figure 1.Dimensions of mild steel (S355JO)
Carburizing materials prepared from a mixture of barium carbonate mixed with
charcoal in the ratio of 2:3 respectively.
Methods
The water quenching method was used. Four specimen were placed in a
crucible and then put to the furnace. The furnace was then heated by use of oil as
a source of fuel to a temperature of 950 oc for about 40 minutes. After the 40
minutes of soaking, the specimen are taken out and placed into cold water for
cooling by the process known as Quenching. When the pieces and the quenching
medium reaches ambient temperature, then they are taken out and eviscerated by
use of the napkin (S. G. Hong, 2010).
The case-hardening process is achieved by placing the specimens in a
stainless cup preventing crucible from carburizing along with the spacimens.The
materials used for carburizing (charcoal and barium carbonate) were put directly
to the specimen, thus reducing them. The stainless cup then put to the crucible in
The sample of cylindrical mild steel rod with the following measurements,
upper diameter of 36mm, lower diameter of 26mm,length of 95mm and 61mm
length of the thinner part as shown below (Adetunji O. R., 2011).
Figure 1.Dimensions of mild steel (S355JO)
Carburizing materials prepared from a mixture of barium carbonate mixed with
charcoal in the ratio of 2:3 respectively.
Methods
The water quenching method was used. Four specimen were placed in a
crucible and then put to the furnace. The furnace was then heated by use of oil as
a source of fuel to a temperature of 950 oc for about 40 minutes. After the 40
minutes of soaking, the specimen are taken out and placed into cold water for
cooling by the process known as Quenching. When the pieces and the quenching
medium reaches ambient temperature, then they are taken out and eviscerated by
use of the napkin (S. G. Hong, 2010).
The case-hardening process is achieved by placing the specimens in a
stainless cup preventing crucible from carburizing along with the spacimens.The
materials used for carburizing (charcoal and barium carbonate) were put directly
to the specimen, thus reducing them. The stainless cup then put to the crucible in
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
the furnace and then heated for 40minutes at a temperature of 950oC.The salt
solution was prepared and then hot specimen placed inside the solution to cool.
This process of heating metals in charcoal and barium carbonate is to increase the
surface hardness in respect to the core. The carbon deposit results to increased
hardness of the metal. This process of hardening the surface of a metal without
hardening the core is called Case-hardening. There are four types of case-
hardening namely, nitriding, cyaniding, carbon-nitriding and carburizing.
Mechanical properties evaluation
The mechanical properties evaluated includes; hardness, tensile, micro-
structure and impact properties.
Hardness testing
The specimen hardness were measured by the method called Rockwell hardness
testing. The procedure adopted includes;
i. Inserting the brale indenter into the machine and adjusted it to 100kg.
ii. 10kg minor load applied to seat of the measured piece.
iii. Major load applied and depth indention recorded automatically in terms of
random hardness number. The dial consists of 100 divisions in which each
division relates to a dispersion of 0.002mm.The value of hardness
obtained then converted into a scale using standard converter (chart).
Testing of Tensile strength
The specimen were treated in UTS machine to obtain the percentage of
elongation, yield strength, and ultimate tensile strength. The procedure used
includes;
the furnace and then heated for 40minutes at a temperature of 950oC.The salt
solution was prepared and then hot specimen placed inside the solution to cool.
This process of heating metals in charcoal and barium carbonate is to increase the
surface hardness in respect to the core. The carbon deposit results to increased
hardness of the metal. This process of hardening the surface of a metal without
hardening the core is called Case-hardening. There are four types of case-
hardening namely, nitriding, cyaniding, carbon-nitriding and carburizing.
Mechanical properties evaluation
The mechanical properties evaluated includes; hardness, tensile, micro-
structure and impact properties.
Hardness testing
The specimen hardness were measured by the method called Rockwell hardness
testing. The procedure adopted includes;
i. Inserting the brale indenter into the machine and adjusted it to 100kg.
ii. 10kg minor load applied to seat of the measured piece.
iii. Major load applied and depth indention recorded automatically in terms of
random hardness number. The dial consists of 100 divisions in which each
division relates to a dispersion of 0.002mm.The value of hardness
obtained then converted into a scale using standard converter (chart).
Testing of Tensile strength
The specimen were treated in UTS machine to obtain the percentage of
elongation, yield strength, and ultimate tensile strength. The procedure used
includes;
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
i. Measuring of the specimen’s cross section area by use of electronic caliper
and the gauge distance calculated.
ii. Fixing the distance between the jaws of the machine and gauge distance of
the specimen.
iii. Specimen was gripped to the holder’s jaw.
iv. Maximum load set at 150KN.
v. Specimen loaded till it fails
vi. Plotting of Load to displacement diagram by use of a software. From the
diagram data is obtained which helps in calculation of yield strength,
percentage elongation and tensile strength.by using the following formula
(W. Yin, 2012):
Percentage elongation= Change∈gauge length
initial gauge length x 100
Yield strength= Load at 0.2 % offset
Initial cross sectional area
Tensile strength = Maximumload
Initial cross section area
Results
Chemical analysis results of mild steel is as shown in the tables below;
Element C Si Mn P S Cr Ni Mo
% 0.142 0.189 0.91 0.041 0.042 0.134 0.111 0.033
Elemen
t
Al Cu Co Ti Nb V W Pb
i. Measuring of the specimen’s cross section area by use of electronic caliper
and the gauge distance calculated.
ii. Fixing the distance between the jaws of the machine and gauge distance of
the specimen.
iii. Specimen was gripped to the holder’s jaw.
iv. Maximum load set at 150KN.
v. Specimen loaded till it fails
vi. Plotting of Load to displacement diagram by use of a software. From the
diagram data is obtained which helps in calculation of yield strength,
percentage elongation and tensile strength.by using the following formula
(W. Yin, 2012):
Percentage elongation= Change∈gauge length
initial gauge length x 100
Yield strength= Load at 0.2 % offset
Initial cross sectional area
Tensile strength = Maximumload
Initial cross section area
Results
Chemical analysis results of mild steel is as shown in the tables below;
Element C Si Mn P S Cr Ni Mo
% 0.142 0.189 0.91 0.041 0.042 0.134 0.111 0.033
Elemen
t
Al Cu Co Ti Nb V W Pb
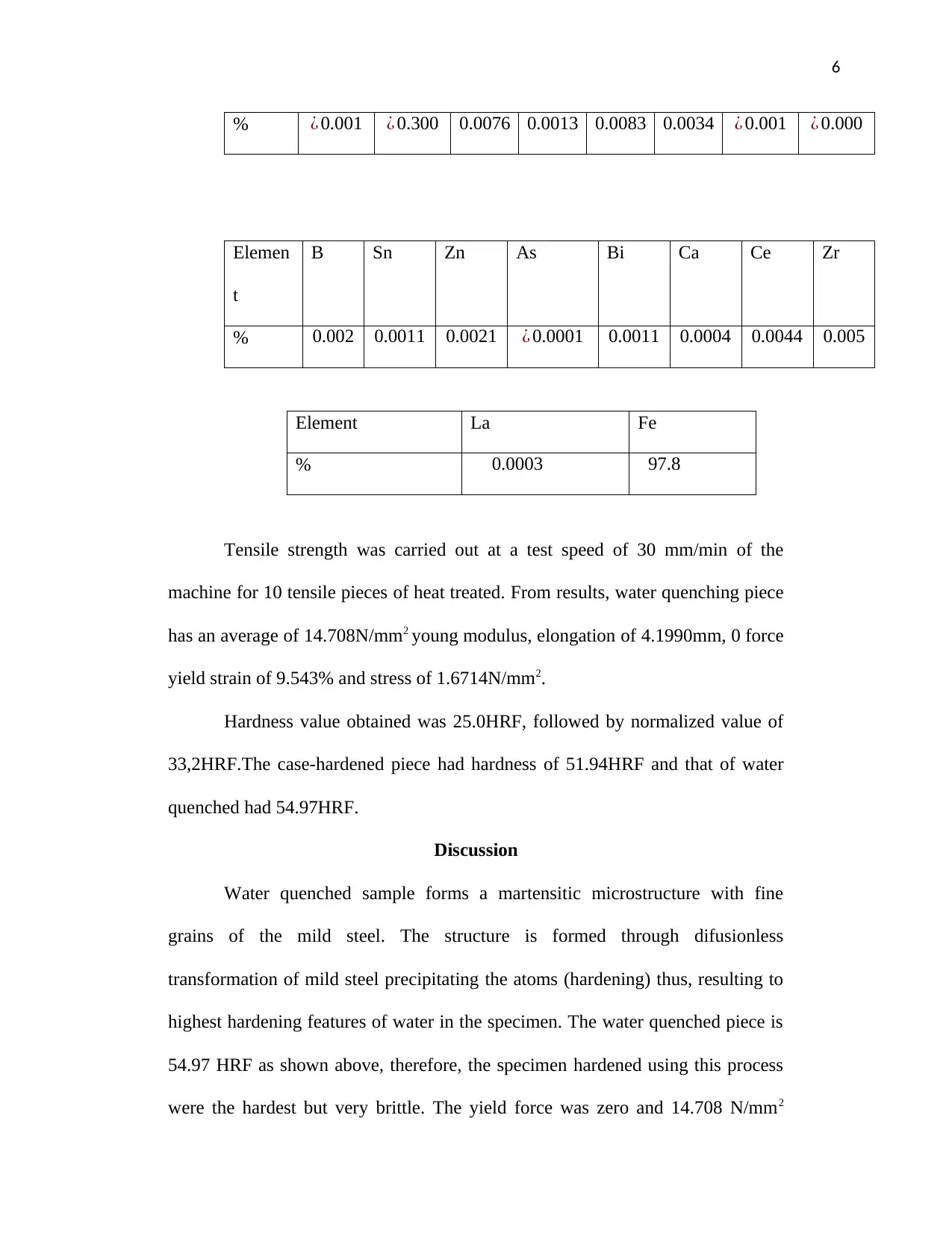
6
% ¿ 0.001 ¿ 0.300 0.0076 0.0013 0.0083 0.0034 ¿ 0.001 ¿ 0.000
Elemen
t
B Sn Zn As Bi Ca Ce Zr
% 0.002 0.0011 0.0021 ¿ 0.0001 0.0011 0.0004 0.0044 0.005
Element La Fe
% 0.0003 97.8
Tensile strength was carried out at a test speed of 30 mm/min of the
machine for 10 tensile pieces of heat treated. From results, water quenching piece
has an average of 14.708N/mm2 young modulus, elongation of 4.1990mm, 0 force
yield strain of 9.543% and stress of 1.6714N/mm2.
Hardness value obtained was 25.0HRF, followed by normalized value of
33,2HRF.The case-hardened piece had hardness of 51.94HRF and that of water
quenched had 54.97HRF.
Discussion
Water quenched sample forms a martensitic microstructure with fine
grains of the mild steel. The structure is formed through difusionless
transformation of mild steel precipitating the atoms (hardening) thus, resulting to
highest hardening features of water in the specimen. The water quenched piece is
54.97 HRF as shown above, therefore, the specimen hardened using this process
were the hardest but very brittle. The yield force was zero and 14.708 N/mm2
% ¿ 0.001 ¿ 0.300 0.0076 0.0013 0.0083 0.0034 ¿ 0.001 ¿ 0.000
Elemen
t
B Sn Zn As Bi Ca Ce Zr
% 0.002 0.0011 0.0021 ¿ 0.0001 0.0011 0.0004 0.0044 0.005
Element La Fe
% 0.0003 97.8
Tensile strength was carried out at a test speed of 30 mm/min of the
machine for 10 tensile pieces of heat treated. From results, water quenching piece
has an average of 14.708N/mm2 young modulus, elongation of 4.1990mm, 0 force
yield strain of 9.543% and stress of 1.6714N/mm2.
Hardness value obtained was 25.0HRF, followed by normalized value of
33,2HRF.The case-hardened piece had hardness of 51.94HRF and that of water
quenched had 54.97HRF.
Discussion
Water quenched sample forms a martensitic microstructure with fine
grains of the mild steel. The structure is formed through difusionless
transformation of mild steel precipitating the atoms (hardening) thus, resulting to
highest hardening features of water in the specimen. The water quenched piece is
54.97 HRF as shown above, therefore, the specimen hardened using this process
were the hardest but very brittle. The yield force was zero and 14.708 N/mm2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
average young modulus. This concludes that water quenched (for 40 minutes at a
temperature of 950oC) results to highest hardness, with marten sic microstructure.
Four specimen were placed in a crucible and then put to the furnace. The furnace
was then heated by use of oil as a source of fuel to a temperature of 950 oc for
about 40 minutes. After the 40 minutes of soaking, the specimen are taken out and
placed into cold water for cooling by the process known as Quenching.
Case–hardening sample was heated in a stainless cup that consists of
barium carbonate and charcoal for 40 minutes at a temperature of 950oC.The
barium carbonate makes the gas produced to be available in the earlier reaction
stages. The source of the gas produced (carbon dioxide) is the charcoal. The mild
steel surface absorbs carbon at a rapid rate. The specimen then it is cooled in salt
water forming marten site structure with mild steel grains resulting to cubic
tetragonal structure. The case –hardened piece have little ductility when compared
to the water-quenched piece. Rise in carbon content is 0.188 resulting to harder
surface than the core. The longer carburization cycle leads to greater depth, high
wear resistance and surface hardness.
Conclusion
Hardening process induces hardness property to the metal rod. Tempering
process soften and improves the ductility of the metal stress relief reducing the
brittleness and making the mild steel tough to resist fatigue and shock. Case
hardening hardens the surface of the metal and not the core section part. The case
hardening types includes nitriding, carburizing, cyaniding and carbon-nitriding.
Reactivity and nature of carbon affects the mechanical properties and resistance
average young modulus. This concludes that water quenched (for 40 minutes at a
temperature of 950oC) results to highest hardness, with marten sic microstructure.
Four specimen were placed in a crucible and then put to the furnace. The furnace
was then heated by use of oil as a source of fuel to a temperature of 950 oc for
about 40 minutes. After the 40 minutes of soaking, the specimen are taken out and
placed into cold water for cooling by the process known as Quenching.
Case–hardening sample was heated in a stainless cup that consists of
barium carbonate and charcoal for 40 minutes at a temperature of 950oC.The
barium carbonate makes the gas produced to be available in the earlier reaction
stages. The source of the gas produced (carbon dioxide) is the charcoal. The mild
steel surface absorbs carbon at a rapid rate. The specimen then it is cooled in salt
water forming marten site structure with mild steel grains resulting to cubic
tetragonal structure. The case –hardened piece have little ductility when compared
to the water-quenched piece. Rise in carbon content is 0.188 resulting to harder
surface than the core. The longer carburization cycle leads to greater depth, high
wear resistance and surface hardness.
Conclusion
Hardening process induces hardness property to the metal rod. Tempering
process soften and improves the ductility of the metal stress relief reducing the
brittleness and making the mild steel tough to resist fatigue and shock. Case
hardening hardens the surface of the metal and not the core section part. The case
hardening types includes nitriding, carburizing, cyaniding and carbon-nitriding.
Reactivity and nature of carbon affects the mechanical properties and resistance
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
of the mild steel. Water quenched sample forms a martensitic microstructure with
fine grains of the mild steel. The structure is formed through difusionless
transformation of mild steel precipitating the atoms (hardening) thus, resulting to
highest hardening features of water in the specimen.
The water quenched piece is 54.97 HRF, therefore, the specimen hardened
using this process were the hardest but very brittle. The yield force was zero and
14.708 N/mm2 average young modulus. This concludes that water quenched (for
40 minutes at a temperature of 950oC) results to highest hardness, with marten sic
microstructure. The source of the gas produced in case-hardening (carbon
dioxide) is the charcoal. The mild steel surface absorbs carbon at a rapid rate. The
specimen then it is cooled in salt water forming marten site structure with mild
steel grains resulting to cubic tetragonal structure. The case –hardened piece have
little ductility when compared to the water-quenched piece. Rise in carbon content
is 0.188 resulting to harder surface than the core. The longer carburization cycle
leads to greater depth, high wear resistance and surface hardness. Case-hardening
process is achieved by placing the specimens in a stainless cup preventing
crucible from carburizing along with the spacimens.The materials used for
carburizing (charcoal and barium carbonate) were put directly to the specimen,
thus reducing them.
of the mild steel. Water quenched sample forms a martensitic microstructure with
fine grains of the mild steel. The structure is formed through difusionless
transformation of mild steel precipitating the atoms (hardening) thus, resulting to
highest hardening features of water in the specimen.
The water quenched piece is 54.97 HRF, therefore, the specimen hardened
using this process were the hardest but very brittle. The yield force was zero and
14.708 N/mm2 average young modulus. This concludes that water quenched (for
40 minutes at a temperature of 950oC) results to highest hardness, with marten sic
microstructure. The source of the gas produced in case-hardening (carbon
dioxide) is the charcoal. The mild steel surface absorbs carbon at a rapid rate. The
specimen then it is cooled in salt water forming marten site structure with mild
steel grains resulting to cubic tetragonal structure. The case –hardened piece have
little ductility when compared to the water-quenched piece. Rise in carbon content
is 0.188 resulting to harder surface than the core. The longer carburization cycle
leads to greater depth, high wear resistance and surface hardness. Case-hardening
process is achieved by placing the specimens in a stainless cup preventing
crucible from carburizing along with the spacimens.The materials used for
carburizing (charcoal and barium carbonate) were put directly to the specimen,
thus reducing them.

9
References
Adetunji O. R., A. P. (2011). Effect of Normalizing and Hardening on Mechanical
Properties of Springs. Journal of Minerals and Materials Characterization and
Engineering, 832-835.
Fadara D. A., F. T. (2011). Effect of Heat Treatment on Mechanical Properties and
Microstructure of NST 37- Steel. Akanbi O.Y., 299-308.
S. G. Hong, S. H. (2010). Heat Treatment of Low Carbon Steel. Journal of Material
Research, 784.
W. Yin, X. J. (2012). Measurement of Decarburization of Steel Rods with an
Electromagnetic Sensor using an Analytical Model. London: Springer.
References
Adetunji O. R., A. P. (2011). Effect of Normalizing and Hardening on Mechanical
Properties of Springs. Journal of Minerals and Materials Characterization and
Engineering, 832-835.
Fadara D. A., F. T. (2011). Effect of Heat Treatment on Mechanical Properties and
Microstructure of NST 37- Steel. Akanbi O.Y., 299-308.
S. G. Hong, S. H. (2010). Heat Treatment of Low Carbon Steel. Journal of Material
Research, 784.
W. Yin, X. J. (2012). Measurement of Decarburization of Steel Rods with an
Electromagnetic Sensor using an Analytical Model. London: Springer.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





