Molecular Biology: Tim-3 Targeted Nanoconjugates in Leukemia Therapy
VerifiedAdded on 2023/06/14
|7
|1329
|374
Report
AI Summary
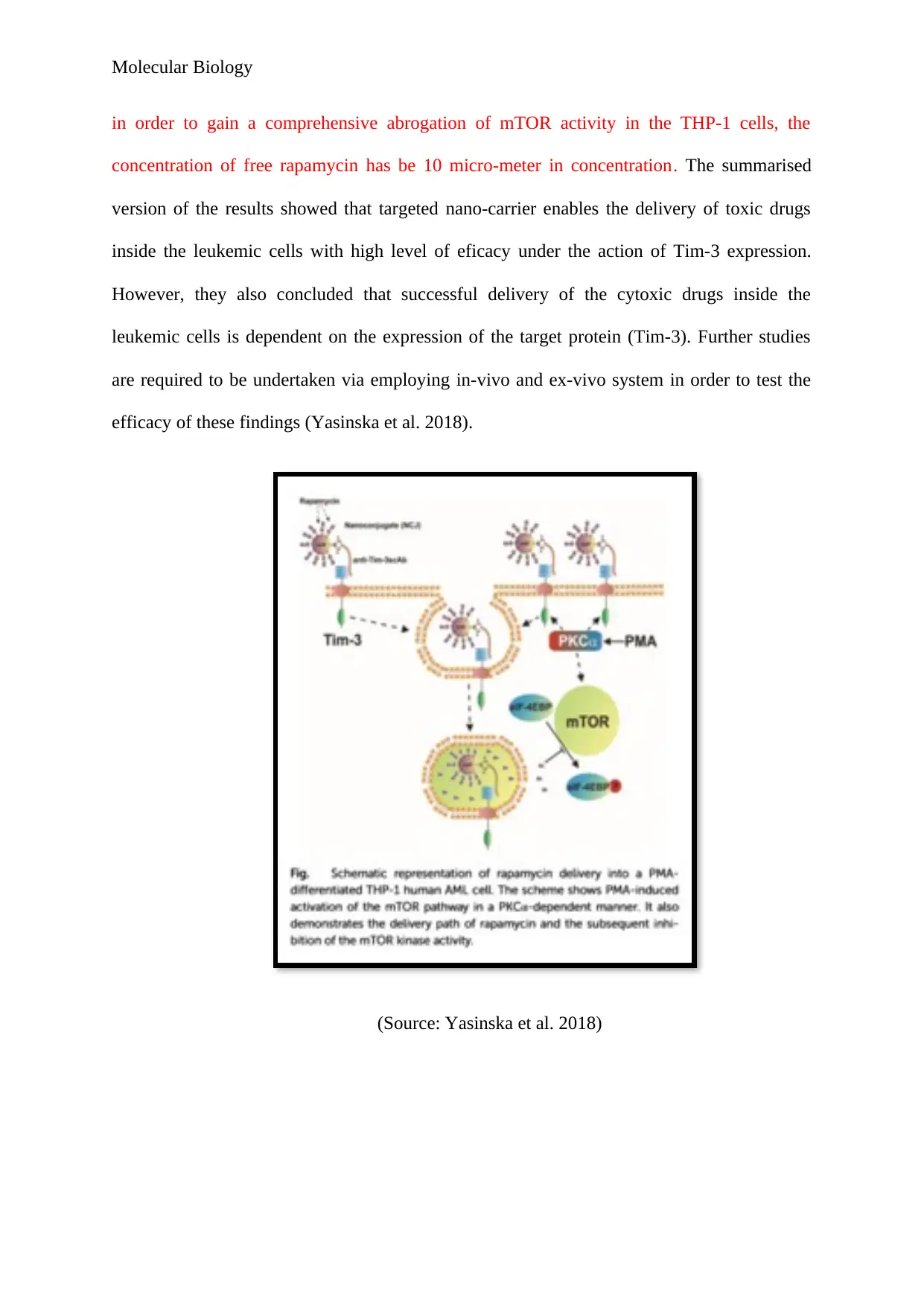
This report delves into the use of citrate-stabilized gold nanoparticles (AuNPs) conjugated with T-cell immunoglobulin and mucin domain 3 (Tim-3) for targeted drug delivery in leukemia treatment, as explored by Yasinska et al. (2018). The study highlights the potential of AuNPs, known for their anti-inflammatory properties, to selectively target malignant leukemic cells while minimizing toxicity to healthy cells. The researchers conjugated anti-Tim3 antibodies with AuNPs using glutathione (GSH) as a linker and then covered the remaining surface with rapamycin, a cytotoxic drug. Spectrophotometry confirmed the formation of the conjugate, and stability tests showed that both the antibody and rapamycin were effectively coupled to the nanoparticles. The study demonstrated that the success of mTOR inhibition in leukemic cells is dependent on the expression of Tim-3 on the cell surface, with the nano-conjugates facilitating the delivery of rapamycin into the cells via endocytosis and GSH cleavage. The report concludes that this targeted nano-carrier system enables effective drug delivery with high efficacy, contingent on the expression of the target protein, Tim-3, and suggests further in-vivo and ex-vivo studies to validate these findings. The study offers significant advancements in cancer therapy by providing a unique approach to targeted drug delivery using nanoparticles and highlights the importance of understanding nano-immune interactions for developing immune-smart cancer nanomedicine.
1 out of 7








![[object Object]](/_next/static/media/star-bottom.7253800d.svg)