EBSU Biotechnology Report: Anti-HIV Treatment with Nanotechnology
VerifiedAdded on 2023/06/01
|25
|5098
|455
Report
AI Summary
This seminar report, submitted to the Department of Biotechnology at Ebonyi State University (EBSU) in partial fulfillment of a Bachelor of Science degree, explores the application of nanotechnology in anti-HIV drug delivery. The report delves into the challenges of HIV/AIDS treatment and highlights the potential of nanomedicine to improve drug efficacy, reduce toxicity, and target specific cell populations. It discusses various nanocarriers, including liposomes, dendrimers, nanoparticles, and polymeric micelles, and their role in delivering antiretroviral (ARV) drugs to anatomical reservoirs where HIV persists. The report also examines the benefits of nanotechnology-based ARV drug delivery systems, such as increased efficiency and reduced adverse effects. It covers the background of HIV, the limitations of HAART, and the potential of nanotechnology to overcome these limitations by improving drug bioavailability, permeability, and intracellular concentration. The report includes a table summarizing different antiretroviral drugs used in nanotherapeutics and provides a detailed discussion on the edification of nanotechnology in the field of drug delivery. This report is a valuable resource for students and researchers interested in the intersection of nanotechnology and HIV treatment.

i
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
COURSE CODE: BTE 483
FEBRUARY, 2023
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
COURSE CODE: BTE 483
FEBRUARY, 2023
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ii
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF
BACHELOR OF SCIENCE ( B. Sc.)
DEGREE IN BIOTECHNOLOGY
COURSE CODE: BTE 483
SUPERVISOR: PROF. E. I. UGWUJA
FEBRUARY, 2023
ANTI-HIV USING NANOTECHNOLOGY
BY
IGWE IFEOMA MODESTA
EBSU/2019/98309
A SEMINAR REPORT SUBMITTED TO THE DEPARTMENT OF
BIOTECHNOLOGY FACULTY OF BIOLOGICAL SCINECE, EBONYI STATE
UNIVERSITY, ABAKALIKI .
IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF
BACHELOR OF SCIENCE ( B. Sc.)
DEGREE IN BIOTECHNOLOGY
COURSE CODE: BTE 483
SUPERVISOR: PROF. E. I. UGWUJA
FEBRUARY, 2023

iii
APPROVAL
This seminar report titled Anti-HIV Using Nanotechnology carried out by Igwe Ifeoma
Modesta with the registration number EBSU/2019/98309 and presented to the Department of
Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department
APPROVAL
This seminar report titled Anti-HIV Using Nanotechnology carried out by Igwe Ifeoma
Modesta with the registration number EBSU/2019/98309 and presented to the Department of
Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

iv
CERTIFICATION
This is to certify that the seminar report titled Anti-HIV Using Nanotechnology carried out
by Igwe Ifeoma Modesta with the registration number EBSU/2019/98309 and presented to
the Department of Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department
CERTIFICATION
This is to certify that the seminar report titled Anti-HIV Using Nanotechnology carried out
by Igwe Ifeoma Modesta with the registration number EBSU/2019/98309 and presented to
the Department of Biotechnology, Faculty of Science, Ebonyi State University Abakaliki.
______________________ ____________________
PROF. E. I. UGWUJA Date
Seminar Supervisor
______________________ ____________________
DR. ALI. FEDRICK Date
Seminar Coordinator
______________________ ____________________
PROF. E. I. UGWUJA Date
Head of department
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

v
ACKNOWLEDGEMENT
I wish to register my profound gratitude to god almighty for the guidance and Grace
throughout my life.
I'm grateful to the entire staff of Biotechnology department for making my learning
interesting educative and Worthwhile.
My special gratitude to my HOD Prof. E. I. Ugwuja for his effort to see that this works or the
light of the day. I appreciate all my amazing lecturers in the department, my wonderful
supervisor, prof. I. E. Ugwuja for his seasoned lectures, to them all, I say we bless Amen.
My regards to my amazing parents Mr and Mrs Francis Igwe, my guidance Mr and Mrs
Chigozie who financially supported my education pursuit, I said amen blessed by God
Almighty and to my beloved siblings, love you all, you are the best.
ACKNOWLEDGEMENT
I wish to register my profound gratitude to god almighty for the guidance and Grace
throughout my life.
I'm grateful to the entire staff of Biotechnology department for making my learning
interesting educative and Worthwhile.
My special gratitude to my HOD Prof. E. I. Ugwuja for his effort to see that this works or the
light of the day. I appreciate all my amazing lecturers in the department, my wonderful
supervisor, prof. I. E. Ugwuja for his seasoned lectures, to them all, I say we bless Amen.
My regards to my amazing parents Mr and Mrs Francis Igwe, my guidance Mr and Mrs
Chigozie who financially supported my education pursuit, I said amen blessed by God
Almighty and to my beloved siblings, love you all, you are the best.

vi
Table of contents
Title page i
Approval ii
Acknowledgment iii
Table of content iv
Abstract v
CHAPTER ONE: INTRODUCTION
1.1 Background of the Study 1
CHAPTER TWO: DISCUSSIONS
2.1 Edification of Nanotechnology In Field Of Drug Delivery 6
2.2 Liposomes 7
2.3 Liposomal ARV Drug Formulation For Anti-HIV Effect 8
2.4 Dendrimer 9
2.4.1 Dendrimer formulation for targeting HIV-AIDS 10
2.4.2 FDA approved dendrimer of AIDS 11
2.5 Nanoparticle 12
2.5.1 Polymeric nanoparticles 12
2.5.2 Solid lipid nanoparticles (SLN) 12
2.5.3 Nano-structured lipid carries (NCL) 13
2.5.4 Inorganic nanoparticles 14
2.6 Polymeric Micelles 14
2.7 Nanocrystal 15
CHAPTER THREE: CONCLUSION
4.1 Conclusion 16
REFERENCE 17
Table of contents
Title page i
Approval ii
Acknowledgment iii
Table of content iv
Abstract v
CHAPTER ONE: INTRODUCTION
1.1 Background of the Study 1
CHAPTER TWO: DISCUSSIONS
2.1 Edification of Nanotechnology In Field Of Drug Delivery 6
2.2 Liposomes 7
2.3 Liposomal ARV Drug Formulation For Anti-HIV Effect 8
2.4 Dendrimer 9
2.4.1 Dendrimer formulation for targeting HIV-AIDS 10
2.4.2 FDA approved dendrimer of AIDS 11
2.5 Nanoparticle 12
2.5.1 Polymeric nanoparticles 12
2.5.2 Solid lipid nanoparticles (SLN) 12
2.5.3 Nano-structured lipid carries (NCL) 13
2.5.4 Inorganic nanoparticles 14
2.6 Polymeric Micelles 14
2.7 Nanocrystal 15
CHAPTER THREE: CONCLUSION
4.1 Conclusion 16
REFERENCE 17
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

vii
ABSTRACT
The biggest challenges of the world in this 21st century is to cure HIV-AIDS .
Nanotechnology is an emerging multidisciplinary field that has the potential to advance the
treatment and prevention of HIV/AIDS radically. The use of nanotechnology for numerous
biomedical applications has become an area of intense research over the last decade.1–10 The
potential advantages of using nanomedicine over conventional HIV therapies include the
capacity to incorporate, encapsulate, or conjugate a variety of drugs to target specific cell
populations and to offer tunable and site-specific drug release .In Present scenario different
antiviral drugs are available in the market to reduce the worse condition and managed
improved survivial rate . In this scenario Nanotechnology based antiretroviral drugs delivery
holds drug and will provide to cure AIDS. Nanotechnology based deliver system
Nanocarriers like Liposomes, dendrimers, Nanoparticles, Polymeric Micelles, Nanovesicles,
Nanoemulsion provide the way to deliver drug to targeting tissue. Nanobased carriers
revolutionized the field of Pharmaceutics and Pharmaco Kinetic’s in target drug delivery. The
present study depicts nano based ARV drug provides increase efficiency with less adverse
effects to control HIV. Like same way we can provide and increase nanobased drug delivery
capacity to other available HIV drugs.
ABSTRACT
The biggest challenges of the world in this 21st century is to cure HIV-AIDS .
Nanotechnology is an emerging multidisciplinary field that has the potential to advance the
treatment and prevention of HIV/AIDS radically. The use of nanotechnology for numerous
biomedical applications has become an area of intense research over the last decade.1–10 The
potential advantages of using nanomedicine over conventional HIV therapies include the
capacity to incorporate, encapsulate, or conjugate a variety of drugs to target specific cell
populations and to offer tunable and site-specific drug release .In Present scenario different
antiviral drugs are available in the market to reduce the worse condition and managed
improved survivial rate . In this scenario Nanotechnology based antiretroviral drugs delivery
holds drug and will provide to cure AIDS. Nanotechnology based deliver system
Nanocarriers like Liposomes, dendrimers, Nanoparticles, Polymeric Micelles, Nanovesicles,
Nanoemulsion provide the way to deliver drug to targeting tissue. Nanobased carriers
revolutionized the field of Pharmaceutics and Pharmaco Kinetic’s in target drug delivery. The
present study depicts nano based ARV drug provides increase efficiency with less adverse
effects to control HIV. Like same way we can provide and increase nanobased drug delivery
capacity to other available HIV drugs.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1
CHAPTER ONE
INTRODUCTION
1.1 Background of the Study
Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) is a
global pandemic and is the leading infectious disease resulting in significant morbidity and
mortality and consequently devastating socioeconomic effects. With the advent of multidrug,
highly active antiretroviral therapy (HAART), the prognosis for HIV-infected patients has
significantly improved; however, it has not eradicated HIV infection, particularly in
sequestered, anatomically privileged sites, such as the brain, testes, gut, liver, kidney, and
secondary lymphoid tissue. HIV most often enters the body via mucosal surfaces and is
transported by dendritic cells to lymphoid organs, where it is then delivered to activated
CD4+ T cells . Productive infection of CD4+ T cells leads to viremia and dissemination of
the virus to other sites in the body. Untreated HIV infection is usually associated with high
plasma viral loads and progressive decline in CD4+ T cells. Antiretroviral drugs inhibit HIV
replication, and treatment with Highly Active Antiretroviral Therapy (HAART), with a
regimen consisting of at least three drugs, from at least two classes of antiretroviral agents,
will suppress plasma viral load to undetectable levels, and lead to recovery of CD4+ T cell
counts. One of the key sources of entry through the mucosal surfaces is the sexual
transmission. The primary path of heterosexual HIV transmission is the female genital tract .
Sexual transmission via the rectal route is also a major issue that, due to its physiology,
renders it more vulnerable to HIV infection (McGowan, 2008). Immune cells, i.e.
macrophages and dendritic cells found in the sub-epithelial layer of the vagina or cervix
mucosa are the main targets of HIV infection. HIV establishes anatomical reservoirs in
lymphoid tissue, the reticuloendothelial system and other sites not shown here. Antiretroviral
drugs do not penetrate these sites adequately. Macrophages and latently infected CD4+ T
cells constitute cellular reservoirs, because antiretroviral drugs do not achieve satisfactory
intracellular concentration within macrophages and antiretrovirals are ineffective against
latent virus, respectively. Potential means of using nanotechnology to combat viral reservoirs
are:( A) Targeted delivery of antiretroviral drugs to the reticuloendothelial system, including
lymphatic tissues ; (B) Targeting the brain; (C) Targeting latently infected CD4+ T cells ; (D)
Achieving optimal intracellular concentration of antiretroviral drugs within macrophages.
CHAPTER ONE
INTRODUCTION
1.1 Background of the Study
Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) is a
global pandemic and is the leading infectious disease resulting in significant morbidity and
mortality and consequently devastating socioeconomic effects. With the advent of multidrug,
highly active antiretroviral therapy (HAART), the prognosis for HIV-infected patients has
significantly improved; however, it has not eradicated HIV infection, particularly in
sequestered, anatomically privileged sites, such as the brain, testes, gut, liver, kidney, and
secondary lymphoid tissue. HIV most often enters the body via mucosal surfaces and is
transported by dendritic cells to lymphoid organs, where it is then delivered to activated
CD4+ T cells . Productive infection of CD4+ T cells leads to viremia and dissemination of
the virus to other sites in the body. Untreated HIV infection is usually associated with high
plasma viral loads and progressive decline in CD4+ T cells. Antiretroviral drugs inhibit HIV
replication, and treatment with Highly Active Antiretroviral Therapy (HAART), with a
regimen consisting of at least three drugs, from at least two classes of antiretroviral agents,
will suppress plasma viral load to undetectable levels, and lead to recovery of CD4+ T cell
counts. One of the key sources of entry through the mucosal surfaces is the sexual
transmission. The primary path of heterosexual HIV transmission is the female genital tract .
Sexual transmission via the rectal route is also a major issue that, due to its physiology,
renders it more vulnerable to HIV infection (McGowan, 2008). Immune cells, i.e.
macrophages and dendritic cells found in the sub-epithelial layer of the vagina or cervix
mucosa are the main targets of HIV infection. HIV establishes anatomical reservoirs in
lymphoid tissue, the reticuloendothelial system and other sites not shown here. Antiretroviral
drugs do not penetrate these sites adequately. Macrophages and latently infected CD4+ T
cells constitute cellular reservoirs, because antiretroviral drugs do not achieve satisfactory
intracellular concentration within macrophages and antiretrovirals are ineffective against
latent virus, respectively. Potential means of using nanotechnology to combat viral reservoirs
are:( A) Targeted delivery of antiretroviral drugs to the reticuloendothelial system, including
lymphatic tissues ; (B) Targeting the brain; (C) Targeting latently infected CD4+ T cells ; (D)
Achieving optimal intracellular concentration of antiretroviral drugs within macrophages.
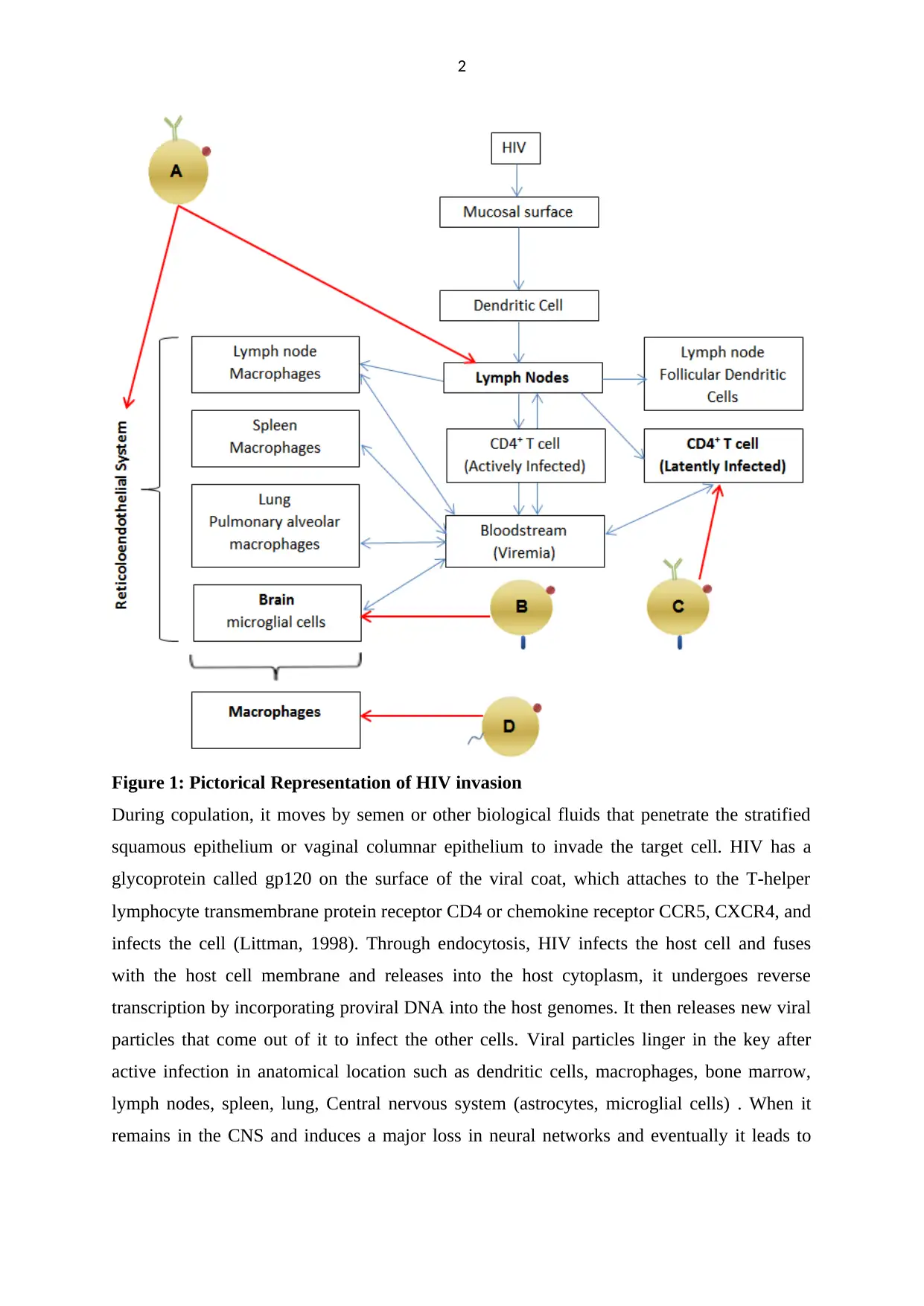
2
Figure 1: Pictorical Representation of HIV invasion
During copulation, it moves by semen or other biological fluids that penetrate the stratified
squamous epithelium or vaginal columnar epithelium to invade the target cell. HIV has a
glycoprotein called gp120 on the surface of the viral coat, which attaches to the T-helper
lymphocyte transmembrane protein receptor CD4 or chemokine receptor CCR5, CXCR4, and
infects the cell (Littman, 1998). Through endocytosis, HIV infects the host cell and fuses
with the host cell membrane and releases into the host cytoplasm, it undergoes reverse
transcription by incorporating proviral DNA into the host genomes. It then releases new viral
particles that come out of it to infect the other cells. Viral particles linger in the key after
active infection in anatomical location such as dendritic cells, macrophages, bone marrow,
lymph nodes, spleen, lung, Central nervous system (astrocytes, microglial cells) . When it
remains in the CNS and induces a major loss in neural networks and eventually it leads to
Figure 1: Pictorical Representation of HIV invasion
During copulation, it moves by semen or other biological fluids that penetrate the stratified
squamous epithelium or vaginal columnar epithelium to invade the target cell. HIV has a
glycoprotein called gp120 on the surface of the viral coat, which attaches to the T-helper
lymphocyte transmembrane protein receptor CD4 or chemokine receptor CCR5, CXCR4, and
infects the cell (Littman, 1998). Through endocytosis, HIV infects the host cell and fuses
with the host cell membrane and releases into the host cytoplasm, it undergoes reverse
transcription by incorporating proviral DNA into the host genomes. It then releases new viral
particles that come out of it to infect the other cells. Viral particles linger in the key after
active infection in anatomical location such as dendritic cells, macrophages, bone marrow,
lymph nodes, spleen, lung, Central nervous system (astrocytes, microglial cells) . When it
remains in the CNS and induces a major loss in neural networks and eventually it leads to
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
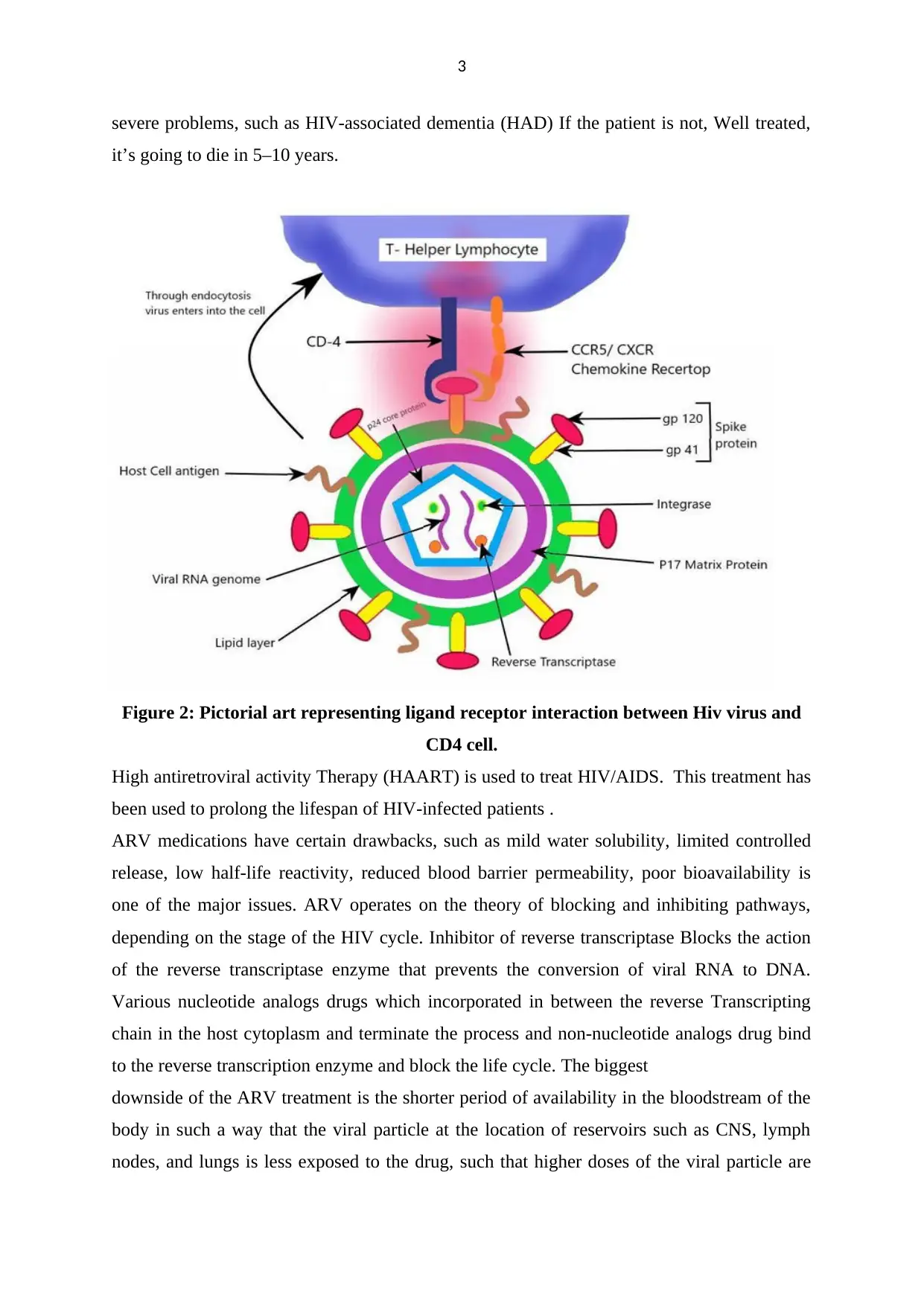
3
severe problems, such as HIV-associated dementia (HAD) If the patient is not, Well treated,
it’s going to die in 5–10 years.
Figure 2: Pictorial art representing ligand receptor interaction between Hiv virus and
CD4 cell.
High antiretroviral activity Therapy (HAART) is used to treat HIV/AIDS. This treatment has
been used to prolong the lifespan of HIV-infected patients .
ARV medications have certain drawbacks, such as mild water solubility, limited controlled
release, low half-life reactivity, reduced blood barrier permeability, poor bioavailability is
one of the major issues. ARV operates on the theory of blocking and inhibiting pathways,
depending on the stage of the HIV cycle. Inhibitor of reverse transcriptase Blocks the action
of the reverse transcriptase enzyme that prevents the conversion of viral RNA to DNA.
Various nucleotide analogs drugs which incorporated in between the reverse Transcripting
chain in the host cytoplasm and terminate the process and non-nucleotide analogs drug bind
to the reverse transcription enzyme and block the life cycle. The biggest
downside of the ARV treatment is the shorter period of availability in the bloodstream of the
body in such a way that the viral particle at the location of reservoirs such as CNS, lymph
nodes, and lungs is less exposed to the drug, such that higher doses of the viral particle are
severe problems, such as HIV-associated dementia (HAD) If the patient is not, Well treated,
it’s going to die in 5–10 years.
Figure 2: Pictorial art representing ligand receptor interaction between Hiv virus and
CD4 cell.
High antiretroviral activity Therapy (HAART) is used to treat HIV/AIDS. This treatment has
been used to prolong the lifespan of HIV-infected patients .
ARV medications have certain drawbacks, such as mild water solubility, limited controlled
release, low half-life reactivity, reduced blood barrier permeability, poor bioavailability is
one of the major issues. ARV operates on the theory of blocking and inhibiting pathways,
depending on the stage of the HIV cycle. Inhibitor of reverse transcriptase Blocks the action
of the reverse transcriptase enzyme that prevents the conversion of viral RNA to DNA.
Various nucleotide analogs drugs which incorporated in between the reverse Transcripting
chain in the host cytoplasm and terminate the process and non-nucleotide analogs drug bind
to the reverse transcription enzyme and block the life cycle. The biggest
downside of the ARV treatment is the shorter period of availability in the bloodstream of the
body in such a way that the viral particle at the location of reservoirs such as CNS, lymph
nodes, and lungs is less exposed to the drug, such that higher doses of the viral particle are
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4
needed for a sustained period of time that develops resistance to the HIV strain. The reservoir
also includes latently infected cells, including CD4+ T-cells, Monocytes, macrophage lineage
carrying incorporated transcription of the provirus silencing within the genome that might
also re-infect the patient due to activation of the proviral genome . In order to resolve such
problems and drawbacks, nano-based drug delivery technologies, nano-medicines, and other
nano-based strategies play a key role in drug effectiveness, drug reactivity, drug target
accuracy, minimizing drug toxicity and negative impacts, and various major challenges
currently facing ARV drugs in the present context.
Summary of antiretroviral drugs used in Hiv nanotherapeutics (table1):
Antiretroviral drug Nanoparticle type
Stavudine (D4T), Methylmethacrylate -sulfoproplyme
Delavirdine (DLV) (MMA-SPM) nanoparticle with grafted RMP-7
Saquinavir (SQV) (RMP-7/MMA-SPMnanopracticles)
Ampenavir Transferrin (Tf)-conjugated
quantum dots
Dapivirine Poly(ε-caprolactone) nanoparticles
Ritonavir Tat-peptide-conjugated pitonavir
nanoparticles
I
Indinavir, ritonavir, Monocyte-derived macrophages-
nanoparticle
atazanavir, and efavirenz interactions
D4T, DLV, SQV Lipids: Compritol 888 ATO,
tripalmitin, and cacao butter
needed for a sustained period of time that develops resistance to the HIV strain. The reservoir
also includes latently infected cells, including CD4+ T-cells, Monocytes, macrophage lineage
carrying incorporated transcription of the provirus silencing within the genome that might
also re-infect the patient due to activation of the proviral genome . In order to resolve such
problems and drawbacks, nano-based drug delivery technologies, nano-medicines, and other
nano-based strategies play a key role in drug effectiveness, drug reactivity, drug target
accuracy, minimizing drug toxicity and negative impacts, and various major challenges
currently facing ARV drugs in the present context.
Summary of antiretroviral drugs used in Hiv nanotherapeutics (table1):
Antiretroviral drug Nanoparticle type
Stavudine (D4T), Methylmethacrylate -sulfoproplyme
Delavirdine (DLV) (MMA-SPM) nanoparticle with grafted RMP-7
Saquinavir (SQV) (RMP-7/MMA-SPMnanopracticles)
Ampenavir Transferrin (Tf)-conjugated
quantum dots
Dapivirine Poly(ε-caprolactone) nanoparticles
Ritonavir Tat-peptide-conjugated pitonavir
nanoparticles
I
Indinavir, ritonavir, Monocyte-derived macrophages-
nanoparticle
atazanavir, and efavirenz interactions
D4T, DLV, SQV Lipids: Compritol 888 ATO,
tripalmitin, and cacao butter

5
stabilized by L-α-
phospatidylcholine, cholesteryl hemisuccinate,
and taurocholate to form
solid lipid nanoparticles
SQV
Nanoparticles with ternary
components of polyethyleneimine,
poly(γ-glutamic acid), and
poly(lactide-co-glycolide acid) (PLGA)
d4T – nucleoside reverse
transcriptase inhibitor
Chitosan-O-isopropyl-5′- O-d4T
monophosphate conjugate
with a phosphoramidate linkage
SQV Tf-conjugated quantum rods
Ritonavir, lopinavir, and efavirenz PLGA nanoparticles
Stavudine Mannose- and galactose-targeted liposome
Efavirenz Mannose-targeted dendrimer
Lamivudine Mannose-targeted dendrimer
Zidovudine Mannose-targeted liposome
Indinavir Liposome-laden macrophages
stabilized by L-α-
phospatidylcholine, cholesteryl hemisuccinate,
and taurocholate to form
solid lipid nanoparticles
SQV
Nanoparticles with ternary
components of polyethyleneimine,
poly(γ-glutamic acid), and
poly(lactide-co-glycolide acid) (PLGA)
d4T – nucleoside reverse
transcriptase inhibitor
Chitosan-O-isopropyl-5′- O-d4T
monophosphate conjugate
with a phosphoramidate linkage
SQV Tf-conjugated quantum rods
Ritonavir, lopinavir, and efavirenz PLGA nanoparticles
Stavudine Mannose- and galactose-targeted liposome
Efavirenz Mannose-targeted dendrimer
Lamivudine Mannose-targeted dendrimer
Zidovudine Mannose-targeted liposome
Indinavir Liposome-laden macrophages
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 25
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.