Neuroscience Systematic Review on Alzheimer's Disease Treatments
VerifiedAdded on 2021/06/15
|19
|4643
|30
Report
AI Summary
This report presents a systematic review of the efficacy of donepezil and bapineuzumab in treating Alzheimer's disease. The review analyzes six articles, following PRISMA guidelines for study selection. The introduction defines Alzheimer's as a neurodegenerative disorder causing memory impairment and dementia, detailing its pathophysiology, including the role of amyloid beta and the amyloid cascade hypothesis. The aetiology section discusses genetic and lifestyle risk factors. The report justifies the research question by highlighting donepezil's efficacy compared to bapineuzumab, which showed limited cognitive improvement. The methodology includes inclusion criteria for double-blind, placebo-controlled trials. The results section provides a table summarizing the findings of the reviewed studies, comparing outcomes such as cognitive scores and nursing home admissions. The review concludes that donepezil demonstrates greater effectiveness in managing Alzheimer's symptoms compared to bapineuzumab, supporting its role as a primary treatment option. The report emphasizes the importance of the research in understanding the treatment landscape of Alzheimer's disease.

Running head: NEUROSCIENCE
Neuroscience Systematic Review
Name of the Student
Name of the University
Author Note
Neuroscience Systematic Review
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1NEUROSCIENCE
Table of Contents
Abstract............................................................................................................................................2
Introduction......................................................................................................................................3
Aetiology.........................................................................................................................................4
Justification for the research question.............................................................................................5
Methods...........................................................................................................................................6
Study selection.............................................................................................................................7
Study characteristics....................................................................................................................8
Risk of bias within studies...........................................................................................................8
Results..............................................................................................................................................8
Discussion......................................................................................................................................15
Conclusion.....................................................................................................................................18
References......................................................................................................................................19
Table of Contents
Abstract............................................................................................................................................2
Introduction......................................................................................................................................3
Aetiology.........................................................................................................................................4
Justification for the research question.............................................................................................5
Methods...........................................................................................................................................6
Study selection.............................................................................................................................7
Study characteristics....................................................................................................................8
Risk of bias within studies...........................................................................................................8
Results..............................................................................................................................................8
Discussion......................................................................................................................................15
Conclusion.....................................................................................................................................18
References......................................................................................................................................19

2NEUROSCIENCE
Abstract
Alzheimer's disease generally refers to a progressive neurological disease that is found to destroy
memory and other essential higher mental functions. Initially, a person suffering from
Alzheimer's disease might notice difficulty in remembering things, in addition to mild confusion.
Eventually, such individuals suffering from the disease might even forget their family members
and acquaintances, thereby undergoing dramatic personality changes. Therefore, Alzheimer's
disease is recognized as the common factor that contributes to dementia that refers to a group of
neurological disorders resulting in loss of social and intellectual skills. In Alzheimer's disease,
the neuronal cells die and degenerate, thereby causing a decline in memory. Current medications
and prevention strategies in AD often encompass administration of drugs such as donepezil, and
bapineuzumab antibody therapy for slowing down the cognitive impairment. This report contains
a systematic review of 6 articles, 3 each for the aforementioned interventions. The guidelines of
a PRISMA chart has been used for selection of the six relevant articles, following which their
major attributes and key findings have been presented in a tabular fashion. An analysis of the
results of the systematic review illustrate on the immediate effects of donepezil, and
bapineuzumab, thereby suggesting that donepezil is more effective in curing the symptoms of
AD, compared to bapineuzumab therapy.
Keywords: Alzheimer’s, disease, memory, cognitive, donepezil, bapineuzumab
Abstract
Alzheimer's disease generally refers to a progressive neurological disease that is found to destroy
memory and other essential higher mental functions. Initially, a person suffering from
Alzheimer's disease might notice difficulty in remembering things, in addition to mild confusion.
Eventually, such individuals suffering from the disease might even forget their family members
and acquaintances, thereby undergoing dramatic personality changes. Therefore, Alzheimer's
disease is recognized as the common factor that contributes to dementia that refers to a group of
neurological disorders resulting in loss of social and intellectual skills. In Alzheimer's disease,
the neuronal cells die and degenerate, thereby causing a decline in memory. Current medications
and prevention strategies in AD often encompass administration of drugs such as donepezil, and
bapineuzumab antibody therapy for slowing down the cognitive impairment. This report contains
a systematic review of 6 articles, 3 each for the aforementioned interventions. The guidelines of
a PRISMA chart has been used for selection of the six relevant articles, following which their
major attributes and key findings have been presented in a tabular fashion. An analysis of the
results of the systematic review illustrate on the immediate effects of donepezil, and
bapineuzumab, thereby suggesting that donepezil is more effective in curing the symptoms of
AD, compared to bapineuzumab therapy.
Keywords: Alzheimer’s, disease, memory, cognitive, donepezil, bapineuzumab
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3NEUROSCIENCE
Introduction
Alzheimer’s disease (AD) is a slowly progressive neurodegenerative brain disorder
extending with memory impairment as a symptom, it is the leading cause of dementia accounting
for approximately 70% of all cases (Fuller and Manford, 1999). According to recent
epidemiological studies, AD affects 20% of human population over 85 year olds.The
pathophysiology of AD is quite complex. Research studies conducted in recent years have
investigated the association between several comprehensive and possible theories that explain
the underlying pathogenesis of the neurological condition. This forms the foundation for
discovering novel therapeutic approaches that can prevent disintegration of the regions of the
brain.Accumulation of amyloid beta serves as a central pathogenic factor for the development of
AD; however, amyloid beta (Aβ) is a soluble peptide produced by the proteolytic cleavage of the
amyloid precursor protein (APP); which is a neuronal transmembrane protein (Johns, 2014). APP
is mainly cleaved by the proteolytic enzyme complex β-Secretase and in a second step the γ-
Secretase is cleaved into soluble, non-pathogenic fragments.This leads to the formation of a
cleavage fragment Aβ ( 1-42), a peptide, found to be of slightly longer length. This peptide
fragments are present in highly aggregated form and show resistance towards the degradation
process, brought about proteolytic enzymes (Morales et al., 2014). This in turn contributes
towards amyloid plaque extracellular aggregation, in combination with oxidative and secondary
toxic process. The toxic process lead to subsequent neuronal damage that is irreversible,
eventually resulting in degeneration of the nerve cells. This is further explained by the amyloid
cascade hypothesis that proposes the role of excess amyloid-beta peptide accumulation in the
onset of AD.
Introduction
Alzheimer’s disease (AD) is a slowly progressive neurodegenerative brain disorder
extending with memory impairment as a symptom, it is the leading cause of dementia accounting
for approximately 70% of all cases (Fuller and Manford, 1999). According to recent
epidemiological studies, AD affects 20% of human population over 85 year olds.The
pathophysiology of AD is quite complex. Research studies conducted in recent years have
investigated the association between several comprehensive and possible theories that explain
the underlying pathogenesis of the neurological condition. This forms the foundation for
discovering novel therapeutic approaches that can prevent disintegration of the regions of the
brain.Accumulation of amyloid beta serves as a central pathogenic factor for the development of
AD; however, amyloid beta (Aβ) is a soluble peptide produced by the proteolytic cleavage of the
amyloid precursor protein (APP); which is a neuronal transmembrane protein (Johns, 2014). APP
is mainly cleaved by the proteolytic enzyme complex β-Secretase and in a second step the γ-
Secretase is cleaved into soluble, non-pathogenic fragments.This leads to the formation of a
cleavage fragment Aβ ( 1-42), a peptide, found to be of slightly longer length. This peptide
fragments are present in highly aggregated form and show resistance towards the degradation
process, brought about proteolytic enzymes (Morales et al., 2014). This in turn contributes
towards amyloid plaque extracellular aggregation, in combination with oxidative and secondary
toxic process. The toxic process lead to subsequent neuronal damage that is irreversible,
eventually resulting in degeneration of the nerve cells. This is further explained by the amyloid
cascade hypothesis that proposes the role of excess amyloid-beta peptide accumulation in the
onset of AD.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4NEUROSCIENCE
Aetiology
Currently known forms of familial Alzheimer’s disease have an autosomal dominant inheritance;
they numerically play a minor role about 5% of cases (Kitazawa et al., 2012). There are
mutations in three genes of importance, their encoded proteins have a significant proportion of
the amyloid processing: presenilin-1 and presenilin-2 (PS1 and PS2) and the gene for APP
(Kitazawa et al., 2012). However, much more frequently occurring than the familial forms is the
sporadic AD (Johns, 2014). Here are so far no-causing genetic factors (mutations or
polymorphisms) are known. However, there are well-founded indications for the presence of
gene polymorphisms that with an increased probability of illness associated, for instance, certain
lifestyle risk factors that can trigger the pathophysiology. A prominent example of such a
susceptibility gene in AD is the gene for apolipoprotein E (ApoE), a Lipid transport protein,
which also functions as a regulator of cholesterol uptake in the cell. Here, the presence of ApoE
is ε 4 allele associated with an increased risk of AD associated, this risk still potentiated in the
presence of atherosclerotic changes (Kitazawa et al., 2012).
The monoclonal antibody bapineuzumab, is designed to bind and eliminate beta-amyloid from
the brain. Bapineuzumab is classified as an IgG1 antibody which binds with soluble and fibrillar
Aβ resulting in activation of microglial phagocytosis leading to production of cytokine. Other
features of Alzheimer's are characteristic changes in brain messengers (so-called
neurotransmitters). Hence, memory disorders, concentration and attention difficulties may also
be due to a deficiency of the neurotransmitter acetylcholine. Acetylcholine plays an important
role in the transmission of impulses between nerve cells as well as between nerve and muscle
cells. Donepezil is a chemically synthesized piperidine: It inhibits reversible and relatively
selective acetylcholinesterase with a mixed competitive and non-competitive mechanism. To
Aetiology
Currently known forms of familial Alzheimer’s disease have an autosomal dominant inheritance;
they numerically play a minor role about 5% of cases (Kitazawa et al., 2012). There are
mutations in three genes of importance, their encoded proteins have a significant proportion of
the amyloid processing: presenilin-1 and presenilin-2 (PS1 and PS2) and the gene for APP
(Kitazawa et al., 2012). However, much more frequently occurring than the familial forms is the
sporadic AD (Johns, 2014). Here are so far no-causing genetic factors (mutations or
polymorphisms) are known. However, there are well-founded indications for the presence of
gene polymorphisms that with an increased probability of illness associated, for instance, certain
lifestyle risk factors that can trigger the pathophysiology. A prominent example of such a
susceptibility gene in AD is the gene for apolipoprotein E (ApoE), a Lipid transport protein,
which also functions as a regulator of cholesterol uptake in the cell. Here, the presence of ApoE
is ε 4 allele associated with an increased risk of AD associated, this risk still potentiated in the
presence of atherosclerotic changes (Kitazawa et al., 2012).
The monoclonal antibody bapineuzumab, is designed to bind and eliminate beta-amyloid from
the brain. Bapineuzumab is classified as an IgG1 antibody which binds with soluble and fibrillar
Aβ resulting in activation of microglial phagocytosis leading to production of cytokine. Other
features of Alzheimer's are characteristic changes in brain messengers (so-called
neurotransmitters). Hence, memory disorders, concentration and attention difficulties may also
be due to a deficiency of the neurotransmitter acetylcholine. Acetylcholine plays an important
role in the transmission of impulses between nerve cells as well as between nerve and muscle
cells. Donepezil is a chemically synthesized piperidine: It inhibits reversible and relatively
selective acetylcholinesterase with a mixed competitive and non-competitive mechanism. To

5NEUROSCIENCE
alleviate the symptoms of Alzheimer's disease and improve memory, donepezil is used as a
selective inhibitor of the enzyme acetylcholinesterase (Kandimalla et al. 2017). When this
enzyme is inhibited in the brain, acetylcholine stays longer on the receptors of the downstream
nerve cell, which makes the signal stronger, so to speak. Thus, the remaining nerve cells can still
communicate with normal intensity.Donepezil is one of the primary medications that belongs to
the class of second generation acetylcholinesterase inhibitors (AChEIs). Other drugs that belong
to this class of inhibitors include galantamine, and rivastigmine. These drugs have been
formulated with the aim using in palliative treatment of patients suffering from AD, during the
early 1980s, with discovery of the association of AD with deficit in the central cholinergic
neurons (Bartus et al 1982; Whitehouse et al 1982).Tacrine, metrifonate, physostigmine,
velnacrine represent the first generation of AChEIs. Tacrine was the only drug that was able to
reach the market in 1993. However, the transient shelf life of the drug due to a range of
interactions between pharmacokinetic and pharmacodynamicmechanisms prevented its
commercialization (Giacobini, 2006). Thus, donepezil was selected as the mainstay of treatment,
following a closure of tacrine use.
Justification for the research question
Although the IgG1 antibody bapineuzumab plays an important role in binding the
soluble, fibrillar Aβ, thereby inducing cytokine production and phagocytosis of the microglia, it
failed to show expected cognitive improvement among patients in two clinical trials. No
significant effects were observed upon implementation of this antibody therapy, even when the
key biomarkers for AD such as, hyperphosphorylated tau proteins and amyloid plaques were
lowered. The efficacy and safety for this antibody was also investigated among patients suffering
from moderate or mild AD. However, the trial failed to show any significant effects on amyloid
alleviate the symptoms of Alzheimer's disease and improve memory, donepezil is used as a
selective inhibitor of the enzyme acetylcholinesterase (Kandimalla et al. 2017). When this
enzyme is inhibited in the brain, acetylcholine stays longer on the receptors of the downstream
nerve cell, which makes the signal stronger, so to speak. Thus, the remaining nerve cells can still
communicate with normal intensity.Donepezil is one of the primary medications that belongs to
the class of second generation acetylcholinesterase inhibitors (AChEIs). Other drugs that belong
to this class of inhibitors include galantamine, and rivastigmine. These drugs have been
formulated with the aim using in palliative treatment of patients suffering from AD, during the
early 1980s, with discovery of the association of AD with deficit in the central cholinergic
neurons (Bartus et al 1982; Whitehouse et al 1982).Tacrine, metrifonate, physostigmine,
velnacrine represent the first generation of AChEIs. Tacrine was the only drug that was able to
reach the market in 1993. However, the transient shelf life of the drug due to a range of
interactions between pharmacokinetic and pharmacodynamicmechanisms prevented its
commercialization (Giacobini, 2006). Thus, donepezil was selected as the mainstay of treatment,
following a closure of tacrine use.
Justification for the research question
Although the IgG1 antibody bapineuzumab plays an important role in binding the
soluble, fibrillar Aβ, thereby inducing cytokine production and phagocytosis of the microglia, it
failed to show expected cognitive improvement among patients in two clinical trials. No
significant effects were observed upon implementation of this antibody therapy, even when the
key biomarkers for AD such as, hyperphosphorylated tau proteins and amyloid plaques were
lowered. The efficacy and safety for this antibody was also investigated among patients suffering
from moderate or mild AD. However, the trial failed to show any significant effects on amyloid
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6NEUROSCIENCE
load or phospohorylated tau present in the CSF. However, the non-competitive, reversible
piperidine-type acetylcholinesterase inhibitor, donezepil has demonstrated its efficacy and
tolerance at all stages of AD among patients. Owing to the fact that AD involves substantial loss
of cholinergic neurons from the cerebral cortex, frontal and parietal lobes, and other areas of the
brain, this acetylcholinesterase inhibitor inhibits the breakdown of the neurotransmitter
acetylcholine, thereby bringing about an increase in its duration of action and effectiveness.
Therefore, this drug has been found to reverse the severity of cognitive impairment, a major
manifestation of AD, in addition to producing antiamnestic actions. These actions help in
cognitive enhancement and improve creativity, memory and other executive functions. Thus, the
research is based on comparing the effectiveness of the aforementioned two therapeutic
interventions, for gaining a deeper insight on treatment of Alzheimer’s disease.
Methods
The inclusion criteria for this paper comprised of studies conducted double blind, placebo-
controlled randomised controlled trials on Alzheimer’s patients, using mainly the drugs:
Bapineuzumab or Donepezil alone or in association with placebo. The research terms employed
were Bapineuzumab, Alzheimer’s; or Donepezil, and Alzheimer’s. For Bapineuzumab, main
emphasis was placed over clinical trials conducted by the manufacturing pharmaceutical
companies. Analysis was done in April 2018 and most of the research articles incorporated aren’t
more than 10 years old, and were published on PubMed, therefore ensuring quality. Data
analysis was done so by means of different scales presented by authors’ of each respective study.
load or phospohorylated tau present in the CSF. However, the non-competitive, reversible
piperidine-type acetylcholinesterase inhibitor, donezepil has demonstrated its efficacy and
tolerance at all stages of AD among patients. Owing to the fact that AD involves substantial loss
of cholinergic neurons from the cerebral cortex, frontal and parietal lobes, and other areas of the
brain, this acetylcholinesterase inhibitor inhibits the breakdown of the neurotransmitter
acetylcholine, thereby bringing about an increase in its duration of action and effectiveness.
Therefore, this drug has been found to reverse the severity of cognitive impairment, a major
manifestation of AD, in addition to producing antiamnestic actions. These actions help in
cognitive enhancement and improve creativity, memory and other executive functions. Thus, the
research is based on comparing the effectiveness of the aforementioned two therapeutic
interventions, for gaining a deeper insight on treatment of Alzheimer’s disease.
Methods
The inclusion criteria for this paper comprised of studies conducted double blind, placebo-
controlled randomised controlled trials on Alzheimer’s patients, using mainly the drugs:
Bapineuzumab or Donepezil alone or in association with placebo. The research terms employed
were Bapineuzumab, Alzheimer’s; or Donepezil, and Alzheimer’s. For Bapineuzumab, main
emphasis was placed over clinical trials conducted by the manufacturing pharmaceutical
companies. Analysis was done in April 2018 and most of the research articles incorporated aren’t
more than 10 years old, and were published on PubMed, therefore ensuring quality. Data
analysis was done so by means of different scales presented by authors’ of each respective study.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
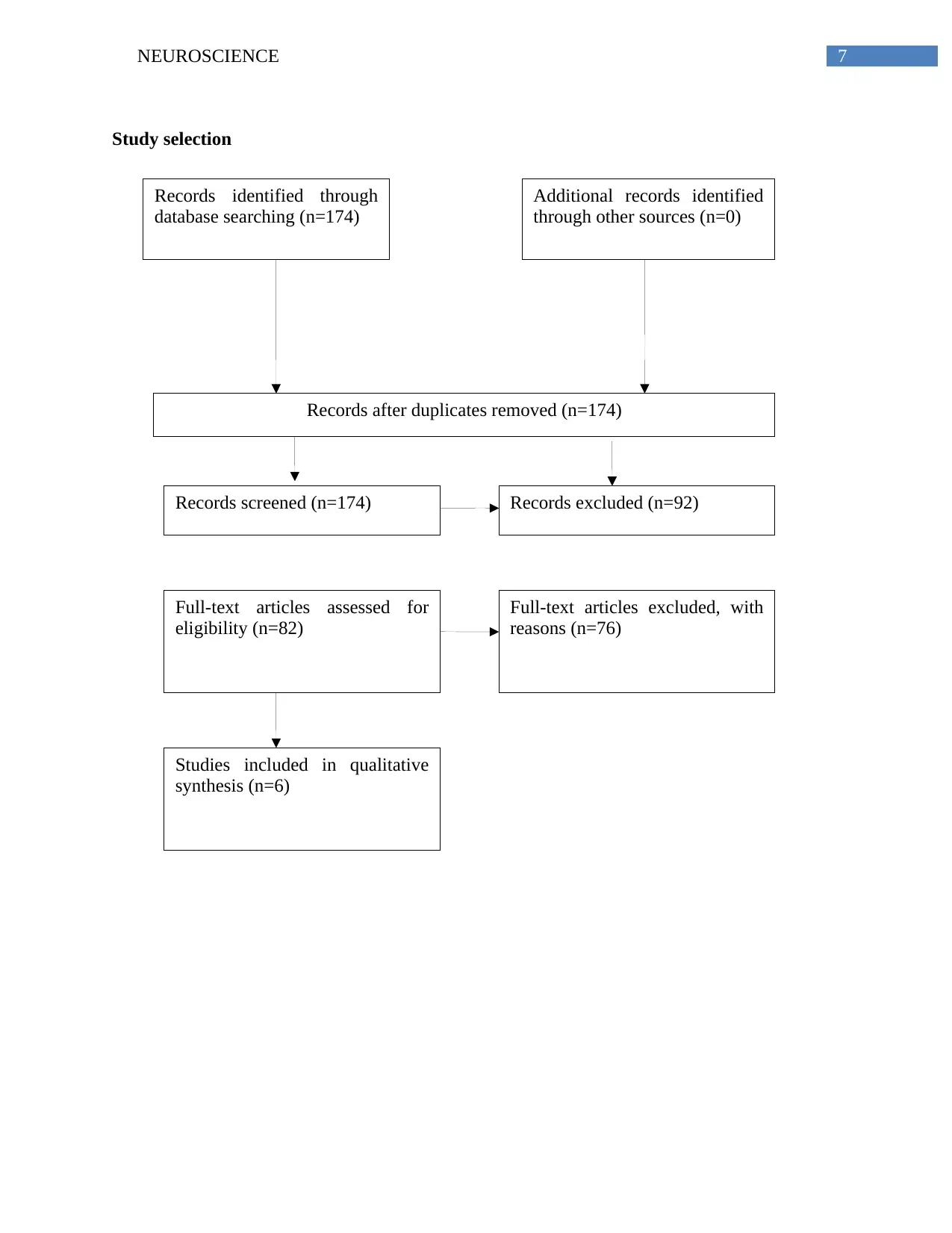
7NEUROSCIENCE
Records identified through
database searching (n=174)
Additional records identified
through other sources (n=0)
Records after duplicates removed (n=174)
Records screened (n=174) Records excluded (n=92)
Full-text articles assessed for
eligibility (n=82)
Full-text articles excluded, with
reasons (n=76)
Studies included in qualitative
synthesis (n=6)
Study selection
Records identified through
database searching (n=174)
Additional records identified
through other sources (n=0)
Records after duplicates removed (n=174)
Records screened (n=174) Records excluded (n=92)
Full-text articles assessed for
eligibility (n=82)
Full-text articles excluded, with
reasons (n=76)
Studies included in qualitative
synthesis (n=6)
Study selection

8NEUROSCIENCE
Study characteristics
Prisma chart statement guidelines was followed during the preparation of this systematic review,
the search retrieved a total of 174 records through medical electronic databases such as PubMed,
18 clinical trials were found using the following research terms: “Bapineuzumab and
Alzheimer’s”; and 156 clinical trials were found using the research query: “Donepezil and
Alzheimer’s”. The filters applied on this search were, clinical trials only, full text, the publication
dates were no more than 10 years old, and animal studies were excluded. No additional studies
were acknowledged through other electronic bases. Furthermore, 174 articles were screened for
eligibility, from which 92 records were excluded for not providing free full access of the text.
Seventy six studies of the full text articles were excluded from the final analysis for the
following reasons; subjects did not have Alzheimer’s disease; duration of treatment was less than
24 weeks; dosage was more than 10mg/d for donepezil or more than 5mg/kg for bapineuzumab;
no abstract; and title did not include the drugs name.
Risk of bias within studies
Bias refers to deviations or systematic errors from the truth, in inferences or results that
might contribute to over-estimation or under-estimation of the intervention effect. It is of utmost
importance to evaluate the risk of bias in all studies, regardless of the variability in the results
and the validity of the studies. It involves assessing the overall strength of the evidences. The
studies might be flawed, even if results are consistent. This will weaken the reliability of the
conclusions about the intervention effect.
Results
Author’s name Number of Intervention Duration Result
Study characteristics
Prisma chart statement guidelines was followed during the preparation of this systematic review,
the search retrieved a total of 174 records through medical electronic databases such as PubMed,
18 clinical trials were found using the following research terms: “Bapineuzumab and
Alzheimer’s”; and 156 clinical trials were found using the research query: “Donepezil and
Alzheimer’s”. The filters applied on this search were, clinical trials only, full text, the publication
dates were no more than 10 years old, and animal studies were excluded. No additional studies
were acknowledged through other electronic bases. Furthermore, 174 articles were screened for
eligibility, from which 92 records were excluded for not providing free full access of the text.
Seventy six studies of the full text articles were excluded from the final analysis for the
following reasons; subjects did not have Alzheimer’s disease; duration of treatment was less than
24 weeks; dosage was more than 10mg/d for donepezil or more than 5mg/kg for bapineuzumab;
no abstract; and title did not include the drugs name.
Risk of bias within studies
Bias refers to deviations or systematic errors from the truth, in inferences or results that
might contribute to over-estimation or under-estimation of the intervention effect. It is of utmost
importance to evaluate the risk of bias in all studies, regardless of the variability in the results
and the validity of the studies. It involves assessing the overall strength of the evidences. The
studies might be flawed, even if results are consistent. This will weaken the reliability of the
conclusions about the intervention effect.
Results
Author’s name Number of Intervention Duration Result
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
9NEUROSCIENCE
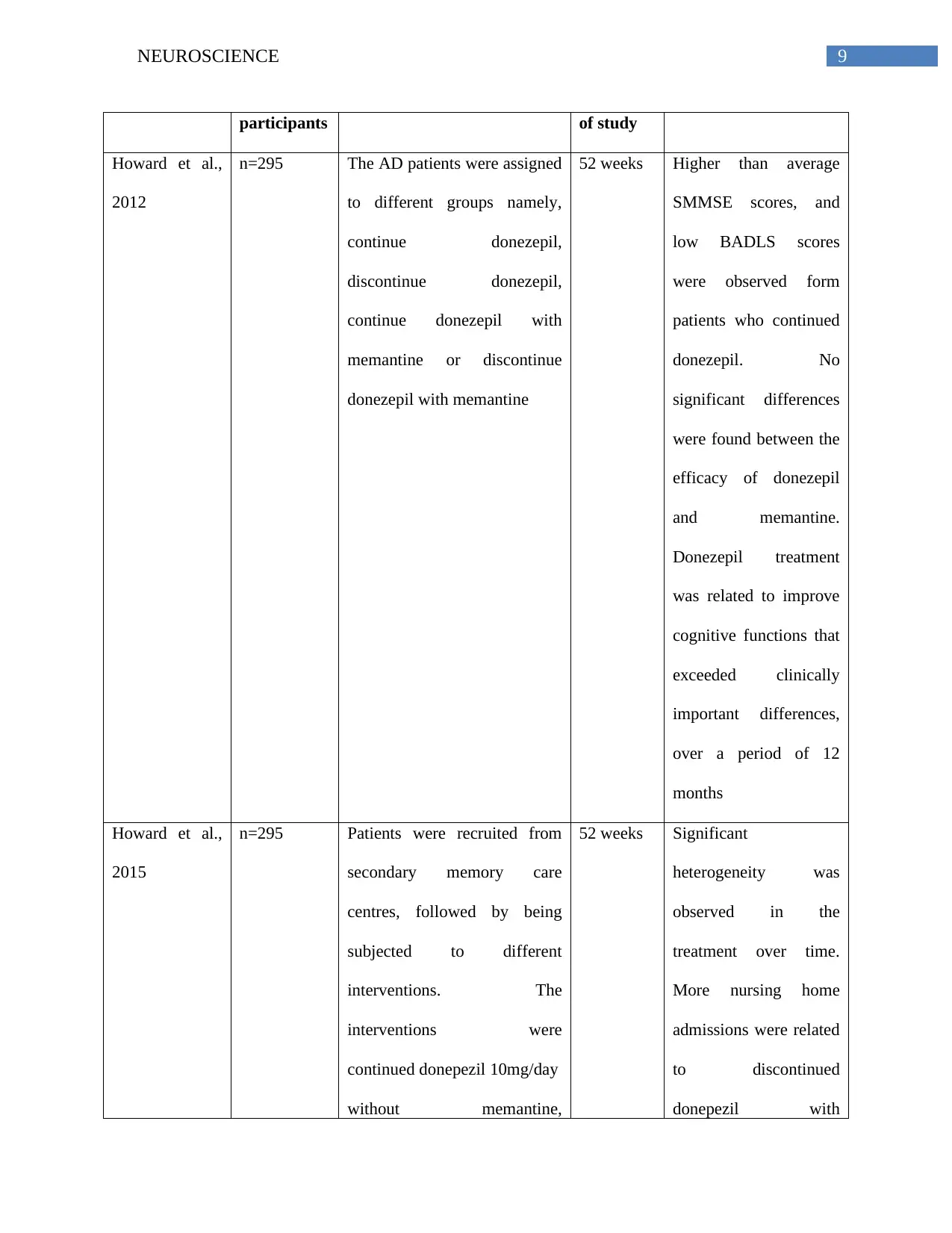
participants of study
Howard et al.,
2012
n=295 The AD patients were assigned
to different groups namely,
continue donezepil,
discontinue donezepil,
continue donezepil with
memantine or discontinue
donezepil with memantine
52 weeks Higher than average
SMMSE scores, and
low BADLS scores
were observed form
patients who continued
donezepil. No
significant differences
were found between the
efficacy of donezepil
and memantine.
Donezepil treatment
was related to improve
cognitive functions that
exceeded clinically
important differences,
over a period of 12
months
Howard et al.,
2015
n=295 Patients were recruited from
secondary memory care
centres, followed by being
subjected to different
interventions. The
interventions were
continued donepezil 10mg/day
without memantine,
52 weeks Significant
heterogeneity was
observed in the
treatment over time.
More nursing home
admissions were related
to discontinued
donepezil with
participants of study
Howard et al.,
2012
n=295 The AD patients were assigned
to different groups namely,
continue donezepil,
discontinue donezepil,
continue donezepil with
memantine or discontinue
donezepil with memantine
52 weeks Higher than average
SMMSE scores, and
low BADLS scores
were observed form
patients who continued
donezepil. No
significant differences
were found between the
efficacy of donezepil
and memantine.
Donezepil treatment
was related to improve
cognitive functions that
exceeded clinically
important differences,
over a period of 12
months
Howard et al.,
2015
n=295 Patients were recruited from
secondary memory care
centres, followed by being
subjected to different
interventions. The
interventions were
continued donepezil 10mg/day
without memantine,
52 weeks Significant
heterogeneity was
observed in the
treatment over time.
More nursing home
admissions were related
to discontinued
donepezil with
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
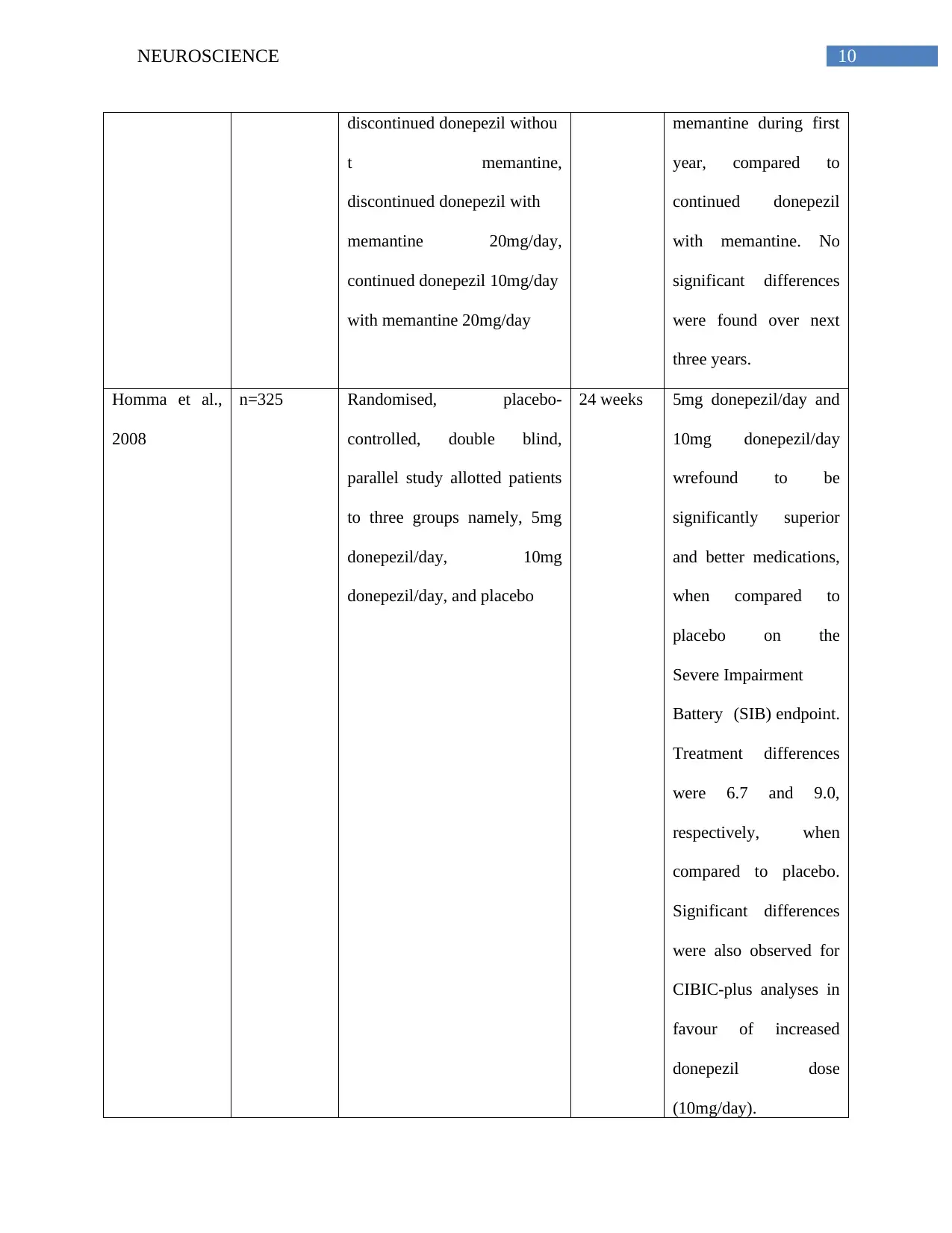
10NEUROSCIENCE
discontinued donepezil withou
t memantine,
discontinued donepezil with
memantine 20mg/day,
continued donepezil 10mg/day
with memantine 20mg/day
memantine during first
year, compared to
continued donepezil
with memantine. No
significant differences
were found over next
three years.
Homma et al.,
2008
n=325 Randomised, placebo-
controlled, double blind,
parallel study allotted patients
to three groups namely, 5mg
donepezil/day, 10mg
donepezil/day, and placebo
24 weeks 5mg donepezil/day and
10mg donepezil/day
wrefound to be
significantly superior
and better medications,
when compared to
placebo on the
Severe Impairment
Battery (SIB) endpoint.
Treatment differences
were 6.7 and 9.0,
respectively, when
compared to placebo.
Significant differences
were also observed for
CIBIC-plus analyses in
favour of increased
donepezil dose
(10mg/day).
discontinued donepezil withou
t memantine,
discontinued donepezil with
memantine 20mg/day,
continued donepezil 10mg/day
with memantine 20mg/day
memantine during first
year, compared to
continued donepezil
with memantine. No
significant differences
were found over next
three years.
Homma et al.,
2008
n=325 Randomised, placebo-
controlled, double blind,
parallel study allotted patients
to three groups namely, 5mg
donepezil/day, 10mg
donepezil/day, and placebo
24 weeks 5mg donepezil/day and
10mg donepezil/day
wrefound to be
significantly superior
and better medications,
when compared to
placebo on the
Severe Impairment
Battery (SIB) endpoint.
Treatment differences
were 6.7 and 9.0,
respectively, when
compared to placebo.
Significant differences
were also observed for
CIBIC-plus analyses in
favour of increased
donepezil dose
(10mg/day).
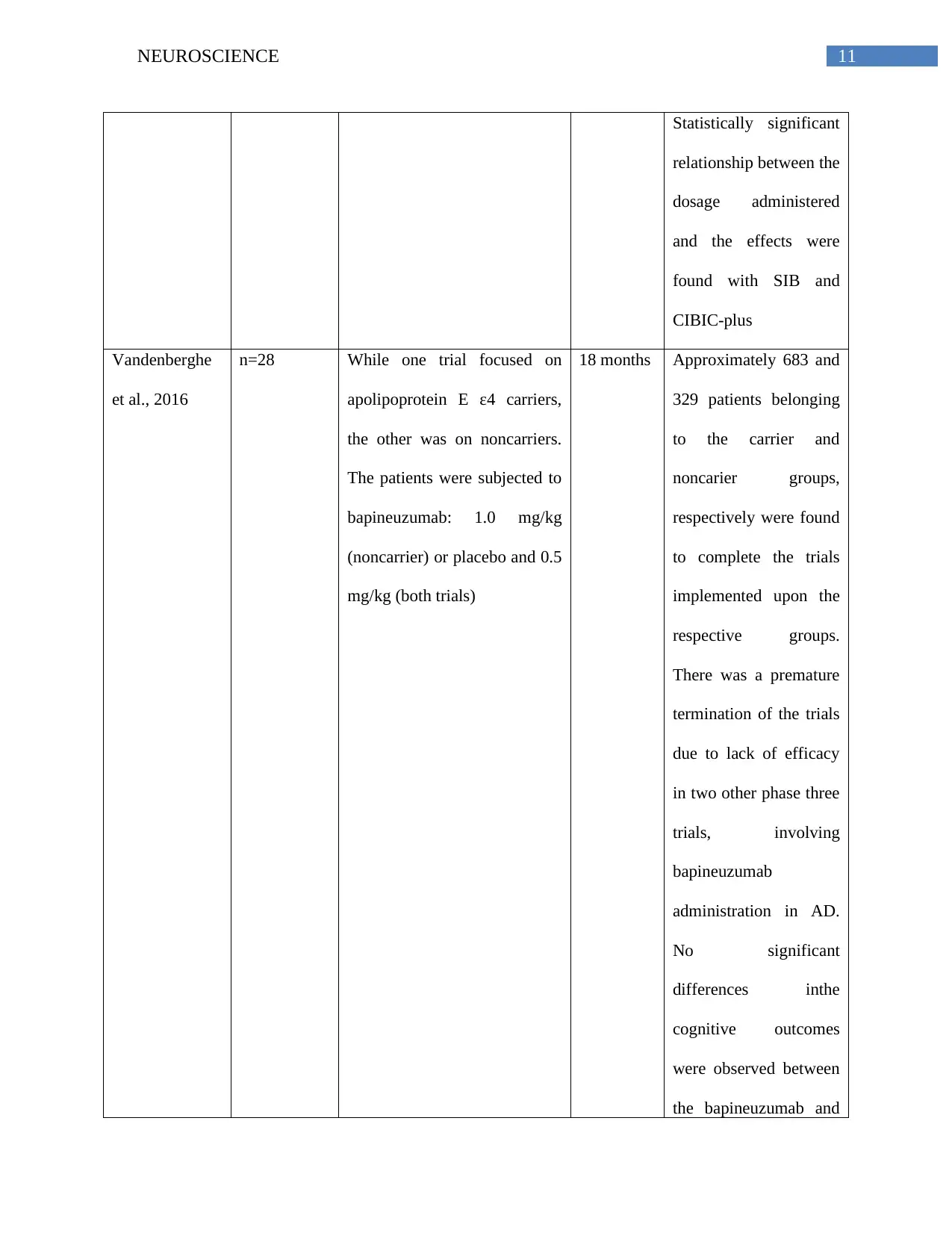
11NEUROSCIENCE
Statistically significant
relationship between the
dosage administered
and the effects were
found with SIB and
CIBIC-plus
Vandenberghe
et al., 2016
n=28 While one trial focused on
apolipoprotein E ε4 carriers,
the other was on noncarriers.
The patients were subjected to
bapineuzumab: 1.0 mg/kg
(noncarrier) or placebo and 0.5
mg/kg (both trials)
18 months Approximately 683 and
329 patients belonging
to the carrier and
noncarier groups,
respectively were found
to complete the trials
implemented upon the
respective groups.
There was a premature
termination of the trials
due to lack of efficacy
in two other phase three
trials, involving
bapineuzumab
administration in AD.
No significant
differences inthe
cognitive outcomes
were observed between
the bapineuzumab and
Statistically significant
relationship between the
dosage administered
and the effects were
found with SIB and
CIBIC-plus
Vandenberghe
et al., 2016
n=28 While one trial focused on
apolipoprotein E ε4 carriers,
the other was on noncarriers.
The patients were subjected to
bapineuzumab: 1.0 mg/kg
(noncarrier) or placebo and 0.5
mg/kg (both trials)
18 months Approximately 683 and
329 patients belonging
to the carrier and
noncarier groups,
respectively were found
to complete the trials
implemented upon the
respective groups.
There was a premature
termination of the trials
due to lack of efficacy
in two other phase three
trials, involving
bapineuzumab
administration in AD.
No significant
differences inthe
cognitive outcomes
were observed between
the bapineuzumab and
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 19
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





