Comprehensive Review: JAMA Article on Psychotic Depression Treatment
VerifiedAdded on 2022/09/02
|5
|2395
|9
Report
AI Summary
This report provides a comprehensive review of the JAMA article titled "Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II randomized clinical trial." The study, conducted at four medical centers between November 2011 and June 2017, investigated the clinical effects of continuing olanzapine in patients with psychotic depression who had responded to a combination of sertraline and olanzapine. The randomized, double-blind, placebo-controlled trial involved 126 participants, with the primary endpoint being the risk of relapse at 36 weeks. The results indicated that continuing olanzapine, in combination with sertraline, significantly reduced the risk of relapse compared to sertraline plus placebo. Secondary endpoints included changes in weight, waist circumference, and metabolic measures. The authors concluded that continuing olanzapine can reduce relapse risk, but potential adverse effects, such as weight gain, must be considered. The study's strengths include its randomized controlled trial design and appropriate statistical analysis, while limitations include the restriction to sertraline and olanzapine and the lack of information on the optimal duration of treatment. The clinical impact suggests the need for guidelines on the continuation or discontinuation of olanzapine in psychotic depression treatment.
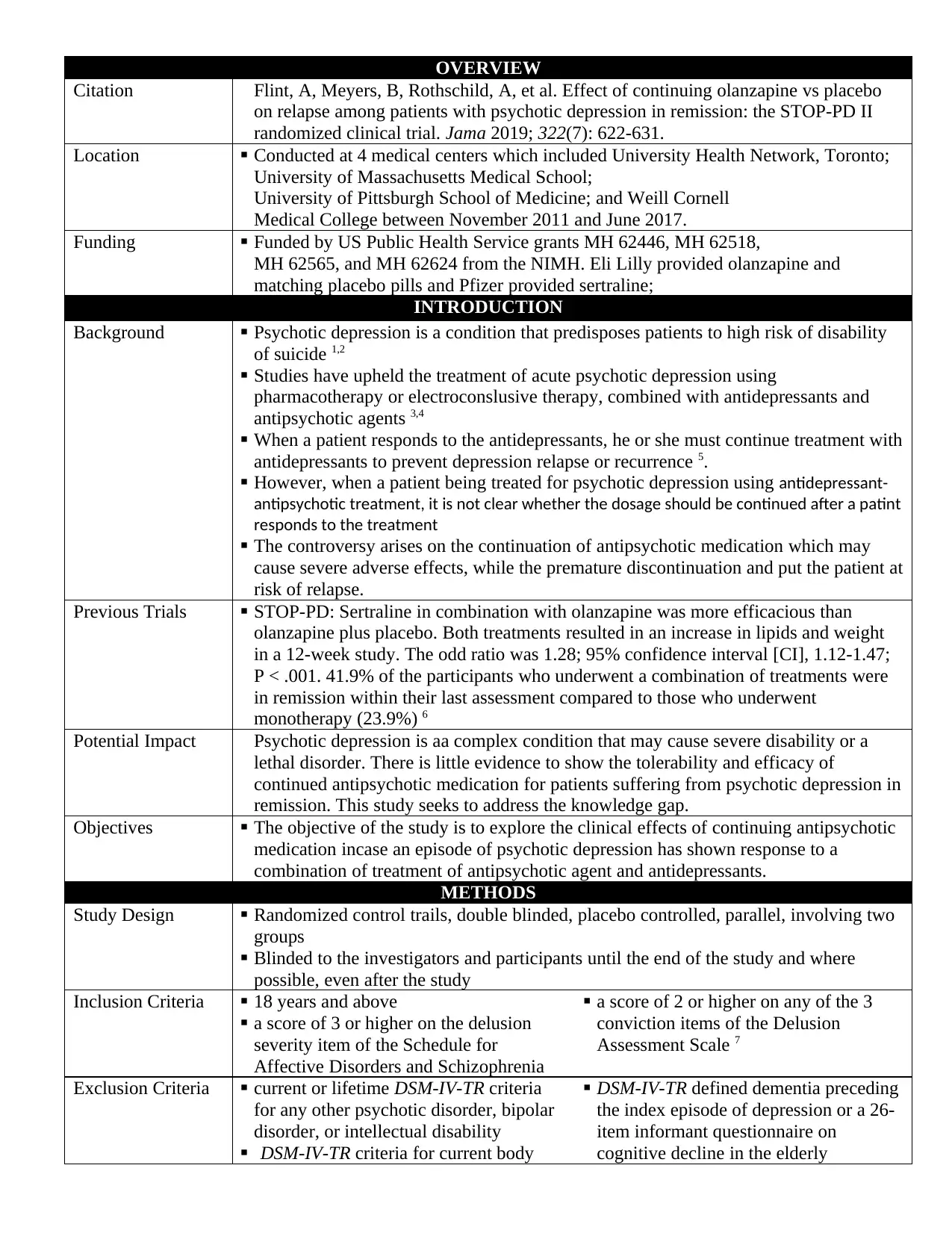
OVERVIEW
Citation Flint, A, Meyers, B, Rothschild, A, et al. Effect of continuing olanzapine vs placebo
on relapse among patients with psychotic depression in remission: the STOP-PD II
randomized clinical trial. Jama 2019; 322(7): 622-631.
Location Conducted at 4 medical centers which included University Health Network, Toronto;
University of Massachusetts Medical School;
University of Pittsburgh School of Medicine; and Weill Cornell
Medical College between November 2011 and June 2017.
Funding Funded by US Public Health Service grants MH 62446, MH 62518,
MH 62565, and MH 62624 from the NIMH. Eli Lilly provided olanzapine and
matching placebo pills and Pfizer provided sertraline;
INTRODUCTION
Background Psychotic depression is a condition that predisposes patients to high risk of disability
of suicide 1,2
Studies have upheld the treatment of acute psychotic depression using
pharmacotherapy or electroconslusive therapy, combined with antidepressants and
antipsychotic agents 3,4
When a patient responds to the antidepressants, he or she must continue treatment with
antidepressants to prevent depression relapse or recurrence 5.
However, when a patient being treated for psychotic depression using antidepressant-
antipsychotic treatment, it is not clear whether the dosage should be continued after a patint
responds to the treatment
The controversy arises on the continuation of antipsychotic medication which may
cause severe adverse effects, while the premature discontinuation and put the patient at
risk of relapse.
Previous Trials STOP-PD: Sertraline in combination with olanzapine was more efficacious than
olanzapine plus placebo. Both treatments resulted in an increase in lipids and weight
in a 12-week study. The odd ratio was 1.28; 95% confidence interval [CI], 1.12-1.47;
P < .001. 41.9% of the participants who underwent a combination of treatments were
in remission within their last assessment compared to those who underwent
monotherapy (23.9%) 6
Potential Impact Psychotic depression is aa complex condition that may cause severe disability or a
lethal disorder. There is little evidence to show the tolerability and efficacy of
continued antipsychotic medication for patients suffering from psychotic depression in
remission. This study seeks to address the knowledge gap.
Objectives The objective of the study is to explore the clinical effects of continuing antipsychotic
medication incase an episode of psychotic depression has shown response to a
combination of treatment of antipsychotic agent and antidepressants.
METHODS
Study Design Randomized control trails, double blinded, placebo controlled, parallel, involving two
groups
Blinded to the investigators and participants until the end of the study and where
possible, even after the study
Inclusion Criteria 18 years and above
a score of 3 or higher on the delusion
severity item of the Schedule for
Affective Disorders and Schizophrenia
a score of 2 or higher on any of the 3
conviction items of the Delusion
Assessment Scale 7
Exclusion Criteria current or lifetime DSM-IV-TR criteria
for any other psychotic disorder, bipolar
disorder, or intellectual disability
DSM-IV-TR criteria for current body
DSM-IV-TR defined dementia preceding
the index episode of depression or a 26-
item informant questionnaire on
cognitive decline in the elderly
Citation Flint, A, Meyers, B, Rothschild, A, et al. Effect of continuing olanzapine vs placebo
on relapse among patients with psychotic depression in remission: the STOP-PD II
randomized clinical trial. Jama 2019; 322(7): 622-631.
Location Conducted at 4 medical centers which included University Health Network, Toronto;
University of Massachusetts Medical School;
University of Pittsburgh School of Medicine; and Weill Cornell
Medical College between November 2011 and June 2017.
Funding Funded by US Public Health Service grants MH 62446, MH 62518,
MH 62565, and MH 62624 from the NIMH. Eli Lilly provided olanzapine and
matching placebo pills and Pfizer provided sertraline;
INTRODUCTION
Background Psychotic depression is a condition that predisposes patients to high risk of disability
of suicide 1,2
Studies have upheld the treatment of acute psychotic depression using
pharmacotherapy or electroconslusive therapy, combined with antidepressants and
antipsychotic agents 3,4
When a patient responds to the antidepressants, he or she must continue treatment with
antidepressants to prevent depression relapse or recurrence 5.
However, when a patient being treated for psychotic depression using antidepressant-
antipsychotic treatment, it is not clear whether the dosage should be continued after a patint
responds to the treatment
The controversy arises on the continuation of antipsychotic medication which may
cause severe adverse effects, while the premature discontinuation and put the patient at
risk of relapse.
Previous Trials STOP-PD: Sertraline in combination with olanzapine was more efficacious than
olanzapine plus placebo. Both treatments resulted in an increase in lipids and weight
in a 12-week study. The odd ratio was 1.28; 95% confidence interval [CI], 1.12-1.47;
P < .001. 41.9% of the participants who underwent a combination of treatments were
in remission within their last assessment compared to those who underwent
monotherapy (23.9%) 6
Potential Impact Psychotic depression is aa complex condition that may cause severe disability or a
lethal disorder. There is little evidence to show the tolerability and efficacy of
continued antipsychotic medication for patients suffering from psychotic depression in
remission. This study seeks to address the knowledge gap.
Objectives The objective of the study is to explore the clinical effects of continuing antipsychotic
medication incase an episode of psychotic depression has shown response to a
combination of treatment of antipsychotic agent and antidepressants.
METHODS
Study Design Randomized control trails, double blinded, placebo controlled, parallel, involving two
groups
Blinded to the investigators and participants until the end of the study and where
possible, even after the study
Inclusion Criteria 18 years and above
a score of 3 or higher on the delusion
severity item of the Schedule for
Affective Disorders and Schizophrenia
a score of 2 or higher on any of the 3
conviction items of the Delusion
Assessment Scale 7
Exclusion Criteria current or lifetime DSM-IV-TR criteria
for any other psychotic disorder, bipolar
disorder, or intellectual disability
DSM-IV-TR criteria for current body
DSM-IV-TR defined dementia preceding
the index episode of depression or a 26-
item informant questionnaire on
cognitive decline in the elderly
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
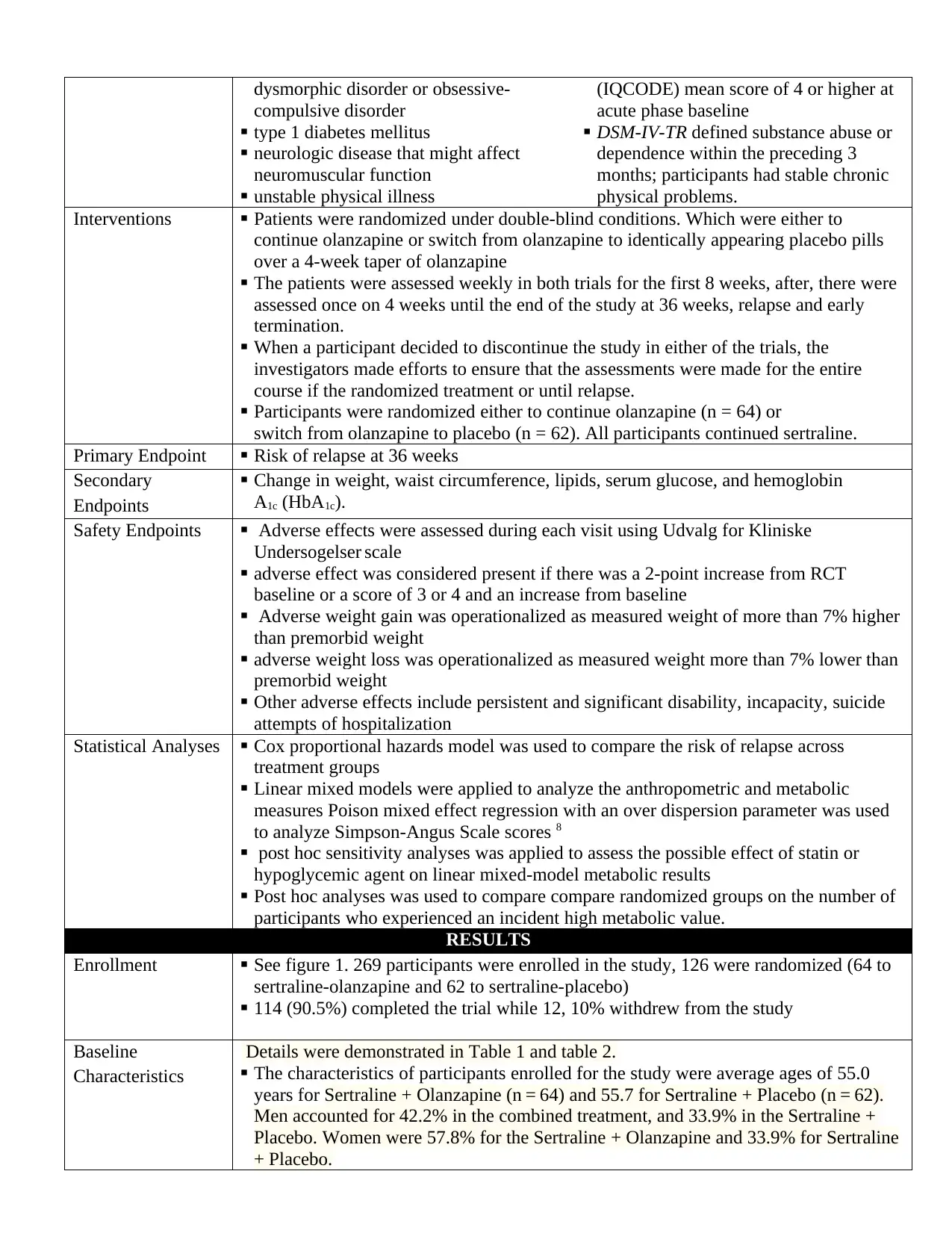
dysmorphic disorder or obsessive-
compulsive disorder
type 1 diabetes mellitus
neurologic disease that might affect
neuromuscular function
unstable physical illness
(IQCODE) mean score of 4 or higher at
acute phase baseline
DSM-IV-TR defined substance abuse or
dependence within the preceding 3
months; participants had stable chronic
physical problems.
Interventions Patients were randomized under double-blind conditions. Which were either to
continue olanzapine or switch from olanzapine to identically appearing placebo pills
over a 4-week taper of olanzapine
The patients were assessed weekly in both trials for the first 8 weeks, after, there were
assessed once on 4 weeks until the end of the study at 36 weeks, relapse and early
termination.
When a participant decided to discontinue the study in either of the trials, the
investigators made efforts to ensure that the assessments were made for the entire
course if the randomized treatment or until relapse.
Participants were randomized either to continue olanzapine (n = 64) or
switch from olanzapine to placebo (n = 62). All participants continued sertraline.
Primary Endpoint Risk of relapse at 36 weeks
Secondary
Endpoints
Change in weight, waist circumference, lipids, serum glucose, and hemoglobin
A1c (HbA1c).
Safety Endpoints Adverse effects were assessed during each visit using Udvalg for Kliniske
Undersogelser scale
adverse effect was considered present if there was a 2-point increase from RCT
baseline or a score of 3 or 4 and an increase from baseline
Adverse weight gain was operationalized as measured weight of more than 7% higher
than premorbid weight
adverse weight loss was operationalized as measured weight more than 7% lower than
premorbid weight
Other adverse effects include persistent and significant disability, incapacity, suicide
attempts of hospitalization
Statistical Analyses Cox proportional hazards model was used to compare the risk of relapse across
treatment groups
Linear mixed models were applied to analyze the anthropometric and metabolic
measures Poison mixed effect regression with an over dispersion parameter was used
to analyze Simpson-Angus Scale scores 8
post hoc sensitivity analyses was applied to assess the possible effect of statin or
hypoglycemic agent on linear mixed-model metabolic results
Post hoc analyses was used to compare compare randomized groups on the number of
participants who experienced an incident high metabolic value.
RESULTS
Enrollment See figure 1. 269 participants were enrolled in the study, 126 were randomized (64 to
sertraline-olanzapine and 62 to sertraline-placebo)
114 (90.5%) completed the trial while 12, 10% withdrew from the study
Baseline
Characteristics
Details were demonstrated in Table 1 and table 2.
The characteristics of participants enrolled for the study were average ages of 55.0
years for Sertraline + Olanzapine (n = 64) and 55.7 for Sertraline + Placebo (n = 62).
Men accounted for 42.2% in the combined treatment, and 33.9% in the Sertraline +
Placebo. Women were 57.8% for the Sertraline + Olanzapine and 33.9% for Sertraline
+ Placebo.
compulsive disorder
type 1 diabetes mellitus
neurologic disease that might affect
neuromuscular function
unstable physical illness
(IQCODE) mean score of 4 or higher at
acute phase baseline
DSM-IV-TR defined substance abuse or
dependence within the preceding 3
months; participants had stable chronic
physical problems.
Interventions Patients were randomized under double-blind conditions. Which were either to
continue olanzapine or switch from olanzapine to identically appearing placebo pills
over a 4-week taper of olanzapine
The patients were assessed weekly in both trials for the first 8 weeks, after, there were
assessed once on 4 weeks until the end of the study at 36 weeks, relapse and early
termination.
When a participant decided to discontinue the study in either of the trials, the
investigators made efforts to ensure that the assessments were made for the entire
course if the randomized treatment or until relapse.
Participants were randomized either to continue olanzapine (n = 64) or
switch from olanzapine to placebo (n = 62). All participants continued sertraline.
Primary Endpoint Risk of relapse at 36 weeks
Secondary
Endpoints
Change in weight, waist circumference, lipids, serum glucose, and hemoglobin
A1c (HbA1c).
Safety Endpoints Adverse effects were assessed during each visit using Udvalg for Kliniske
Undersogelser scale
adverse effect was considered present if there was a 2-point increase from RCT
baseline or a score of 3 or 4 and an increase from baseline
Adverse weight gain was operationalized as measured weight of more than 7% higher
than premorbid weight
adverse weight loss was operationalized as measured weight more than 7% lower than
premorbid weight
Other adverse effects include persistent and significant disability, incapacity, suicide
attempts of hospitalization
Statistical Analyses Cox proportional hazards model was used to compare the risk of relapse across
treatment groups
Linear mixed models were applied to analyze the anthropometric and metabolic
measures Poison mixed effect regression with an over dispersion parameter was used
to analyze Simpson-Angus Scale scores 8
post hoc sensitivity analyses was applied to assess the possible effect of statin or
hypoglycemic agent on linear mixed-model metabolic results
Post hoc analyses was used to compare compare randomized groups on the number of
participants who experienced an incident high metabolic value.
RESULTS
Enrollment See figure 1. 269 participants were enrolled in the study, 126 were randomized (64 to
sertraline-olanzapine and 62 to sertraline-placebo)
114 (90.5%) completed the trial while 12, 10% withdrew from the study
Baseline
Characteristics
Details were demonstrated in Table 1 and table 2.
The characteristics of participants enrolled for the study were average ages of 55.0
years for Sertraline + Olanzapine (n = 64) and 55.7 for Sertraline + Placebo (n = 62).
Men accounted for 42.2% in the combined treatment, and 33.9% in the Sertraline +
Placebo. Women were 57.8% for the Sertraline + Olanzapine and 33.9% for Sertraline
+ Placebo.
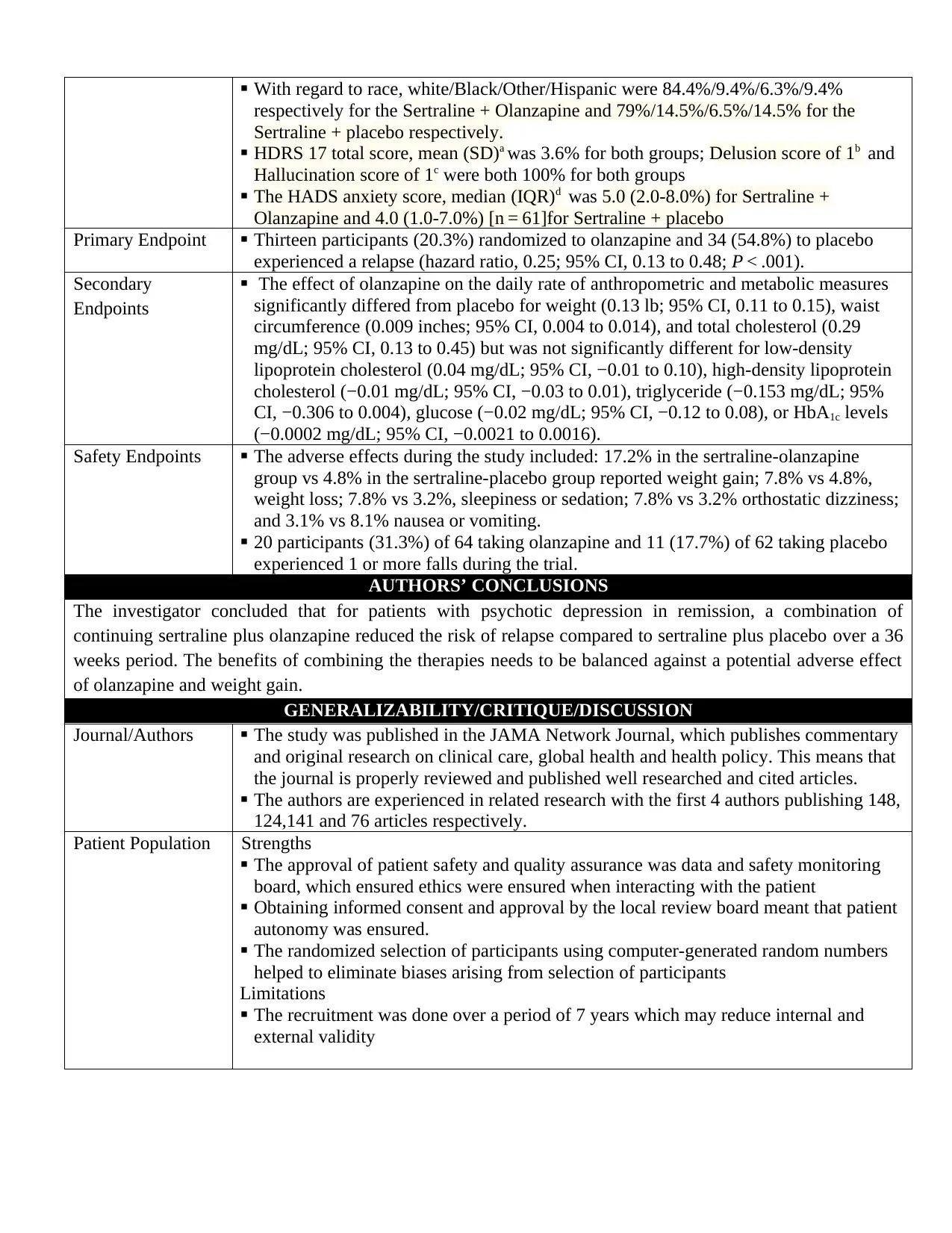
With regard to race, white/Black/Other/Hispanic were 84.4%/9.4%/6.3%/9.4%
respectively for the Sertraline + Olanzapine and 79%/14.5%/6.5%/14.5% for the
Sertraline + placebo respectively.
HDRS 17 total score, mean (SD)a was 3.6% for both groups; Delusion score of 1b and
Hallucination score of 1c were both 100% for both groups
The HADS anxiety score, median (IQR)d was 5.0 (2.0-8.0%) for Sertraline +
Olanzapine and 4.0 (1.0-7.0%) [n = 61]for Sertraline + placebo
Primary Endpoint Thirteen participants (20.3%) randomized to olanzapine and 34 (54.8%) to placebo
experienced a relapse (hazard ratio, 0.25; 95% CI, 0.13 to 0.48; P < .001).
Secondary
Endpoints
The effect of olanzapine on the daily rate of anthropometric and metabolic measures
significantly differed from placebo for weight (0.13 lb; 95% CI, 0.11 to 0.15), waist
circumference (0.009 inches; 95% CI, 0.004 to 0.014), and total cholesterol (0.29
mg/dL; 95% CI, 0.13 to 0.45) but was not significantly different for low-density
lipoprotein cholesterol (0.04 mg/dL; 95% CI, −0.01 to 0.10), high-density lipoprotein
cholesterol (−0.01 mg/dL; 95% CI, −0.03 to 0.01), triglyceride (−0.153 mg/dL; 95%
CI, −0.306 to 0.004), glucose (−0.02 mg/dL; 95% CI, −0.12 to 0.08), or HbA1c levels
(−0.0002 mg/dL; 95% CI, −0.0021 to 0.0016).
Safety Endpoints The adverse effects during the study included: 17.2% in the sertraline-olanzapine
group vs 4.8% in the sertraline-placebo group reported weight gain; 7.8% vs 4.8%,
weight loss; 7.8% vs 3.2%, sleepiness or sedation; 7.8% vs 3.2% orthostatic dizziness;
and 3.1% vs 8.1% nausea or vomiting.
20 participants (31.3%) of 64 taking olanzapine and 11 (17.7%) of 62 taking placebo
experienced 1 or more falls during the trial.
AUTHORS’ CONCLUSIONS
The investigator concluded that for patients with psychotic depression in remission, a combination of
continuing sertraline plus olanzapine reduced the risk of relapse compared to sertraline plus placebo over a 36
weeks period. The benefits of combining the therapies needs to be balanced against a potential adverse effect
of olanzapine and weight gain.
GENERALIZABILITY/CRITIQUE/DISCUSSION
Journal/Authors The study was published in the JAMA Network Journal, which publishes commentary
and original research on clinical care, global health and health policy. This means that
the journal is properly reviewed and published well researched and cited articles.
The authors are experienced in related research with the first 4 authors publishing 148,
124,141 and 76 articles respectively.
Patient Population Strengths
The approval of patient safety and quality assurance was data and safety monitoring
board, which ensured ethics were ensured when interacting with the patient
Obtaining informed consent and approval by the local review board meant that patient
autonomy was ensured.
The randomized selection of participants using computer-generated random numbers
helped to eliminate biases arising from selection of participants
Limitations
The recruitment was done over a period of 7 years which may reduce internal and
external validity
respectively for the Sertraline + Olanzapine and 79%/14.5%/6.5%/14.5% for the
Sertraline + placebo respectively.
HDRS 17 total score, mean (SD)a was 3.6% for both groups; Delusion score of 1b and
Hallucination score of 1c were both 100% for both groups
The HADS anxiety score, median (IQR)d was 5.0 (2.0-8.0%) for Sertraline +
Olanzapine and 4.0 (1.0-7.0%) [n = 61]for Sertraline + placebo
Primary Endpoint Thirteen participants (20.3%) randomized to olanzapine and 34 (54.8%) to placebo
experienced a relapse (hazard ratio, 0.25; 95% CI, 0.13 to 0.48; P < .001).
Secondary
Endpoints
The effect of olanzapine on the daily rate of anthropometric and metabolic measures
significantly differed from placebo for weight (0.13 lb; 95% CI, 0.11 to 0.15), waist
circumference (0.009 inches; 95% CI, 0.004 to 0.014), and total cholesterol (0.29
mg/dL; 95% CI, 0.13 to 0.45) but was not significantly different for low-density
lipoprotein cholesterol (0.04 mg/dL; 95% CI, −0.01 to 0.10), high-density lipoprotein
cholesterol (−0.01 mg/dL; 95% CI, −0.03 to 0.01), triglyceride (−0.153 mg/dL; 95%
CI, −0.306 to 0.004), glucose (−0.02 mg/dL; 95% CI, −0.12 to 0.08), or HbA1c levels
(−0.0002 mg/dL; 95% CI, −0.0021 to 0.0016).
Safety Endpoints The adverse effects during the study included: 17.2% in the sertraline-olanzapine
group vs 4.8% in the sertraline-placebo group reported weight gain; 7.8% vs 4.8%,
weight loss; 7.8% vs 3.2%, sleepiness or sedation; 7.8% vs 3.2% orthostatic dizziness;
and 3.1% vs 8.1% nausea or vomiting.
20 participants (31.3%) of 64 taking olanzapine and 11 (17.7%) of 62 taking placebo
experienced 1 or more falls during the trial.
AUTHORS’ CONCLUSIONS
The investigator concluded that for patients with psychotic depression in remission, a combination of
continuing sertraline plus olanzapine reduced the risk of relapse compared to sertraline plus placebo over a 36
weeks period. The benefits of combining the therapies needs to be balanced against a potential adverse effect
of olanzapine and weight gain.
GENERALIZABILITY/CRITIQUE/DISCUSSION
Journal/Authors The study was published in the JAMA Network Journal, which publishes commentary
and original research on clinical care, global health and health policy. This means that
the journal is properly reviewed and published well researched and cited articles.
The authors are experienced in related research with the first 4 authors publishing 148,
124,141 and 76 articles respectively.
Patient Population Strengths
The approval of patient safety and quality assurance was data and safety monitoring
board, which ensured ethics were ensured when interacting with the patient
Obtaining informed consent and approval by the local review board meant that patient
autonomy was ensured.
The randomized selection of participants using computer-generated random numbers
helped to eliminate biases arising from selection of participants
Limitations
The recruitment was done over a period of 7 years which may reduce internal and
external validity
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
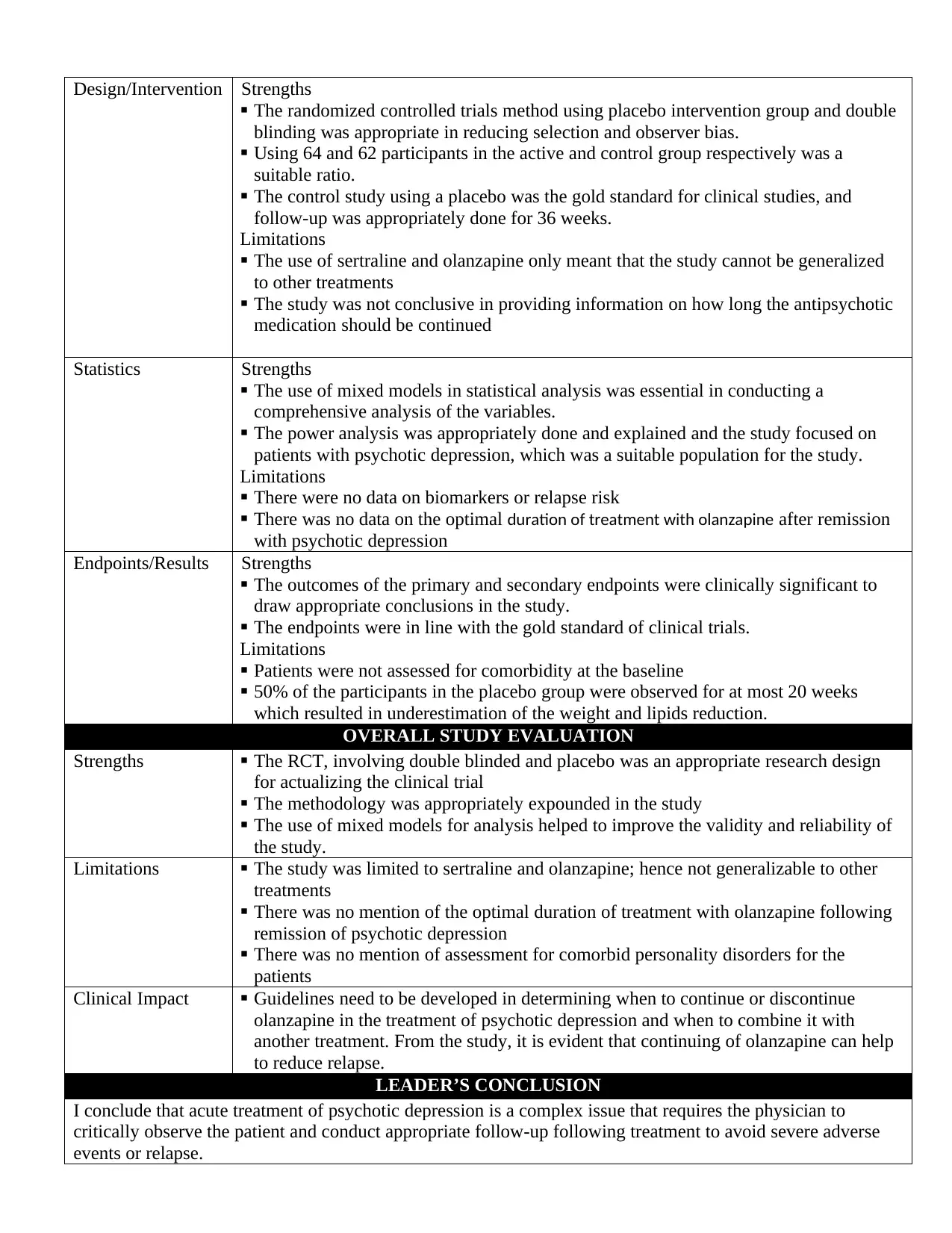
Design/Intervention Strengths
The randomized controlled trials method using placebo intervention group and double
blinding was appropriate in reducing selection and observer bias.
Using 64 and 62 participants in the active and control group respectively was a
suitable ratio.
The control study using a placebo was the gold standard for clinical studies, and
follow-up was appropriately done for 36 weeks.
Limitations
The use of sertraline and olanzapine only meant that the study cannot be generalized
to other treatments
The study was not conclusive in providing information on how long the antipsychotic
medication should be continued
Statistics Strengths
The use of mixed models in statistical analysis was essential in conducting a
comprehensive analysis of the variables.
The power analysis was appropriately done and explained and the study focused on
patients with psychotic depression, which was a suitable population for the study.
Limitations
There were no data on biomarkers or relapse risk
There was no data on the optimal duration of treatment with olanzapine after remission
with psychotic depression
Endpoints/Results Strengths
The outcomes of the primary and secondary endpoints were clinically significant to
draw appropriate conclusions in the study.
The endpoints were in line with the gold standard of clinical trials.
Limitations
Patients were not assessed for comorbidity at the baseline
50% of the participants in the placebo group were observed for at most 20 weeks
which resulted in underestimation of the weight and lipids reduction.
OVERALL STUDY EVALUATION
Strengths The RCT, involving double blinded and placebo was an appropriate research design
for actualizing the clinical trial
The methodology was appropriately expounded in the study
The use of mixed models for analysis helped to improve the validity and reliability of
the study.
Limitations The study was limited to sertraline and olanzapine; hence not generalizable to other
treatments
There was no mention of the optimal duration of treatment with olanzapine following
remission of psychotic depression
There was no mention of assessment for comorbid personality disorders for the
patients
Clinical Impact Guidelines need to be developed in determining when to continue or discontinue
olanzapine in the treatment of psychotic depression and when to combine it with
another treatment. From the study, it is evident that continuing of olanzapine can help
to reduce relapse.
LEADER’S CONCLUSION
I conclude that acute treatment of psychotic depression is a complex issue that requires the physician to
critically observe the patient and conduct appropriate follow-up following treatment to avoid severe adverse
events or relapse.
The randomized controlled trials method using placebo intervention group and double
blinding was appropriate in reducing selection and observer bias.
Using 64 and 62 participants in the active and control group respectively was a
suitable ratio.
The control study using a placebo was the gold standard for clinical studies, and
follow-up was appropriately done for 36 weeks.
Limitations
The use of sertraline and olanzapine only meant that the study cannot be generalized
to other treatments
The study was not conclusive in providing information on how long the antipsychotic
medication should be continued
Statistics Strengths
The use of mixed models in statistical analysis was essential in conducting a
comprehensive analysis of the variables.
The power analysis was appropriately done and explained and the study focused on
patients with psychotic depression, which was a suitable population for the study.
Limitations
There were no data on biomarkers or relapse risk
There was no data on the optimal duration of treatment with olanzapine after remission
with psychotic depression
Endpoints/Results Strengths
The outcomes of the primary and secondary endpoints were clinically significant to
draw appropriate conclusions in the study.
The endpoints were in line with the gold standard of clinical trials.
Limitations
Patients were not assessed for comorbidity at the baseline
50% of the participants in the placebo group were observed for at most 20 weeks
which resulted in underestimation of the weight and lipids reduction.
OVERALL STUDY EVALUATION
Strengths The RCT, involving double blinded and placebo was an appropriate research design
for actualizing the clinical trial
The methodology was appropriately expounded in the study
The use of mixed models for analysis helped to improve the validity and reliability of
the study.
Limitations The study was limited to sertraline and olanzapine; hence not generalizable to other
treatments
There was no mention of the optimal duration of treatment with olanzapine following
remission of psychotic depression
There was no mention of assessment for comorbid personality disorders for the
patients
Clinical Impact Guidelines need to be developed in determining when to continue or discontinue
olanzapine in the treatment of psychotic depression and when to combine it with
another treatment. From the study, it is evident that continuing of olanzapine can help
to reduce relapse.
LEADER’S CONCLUSION
I conclude that acute treatment of psychotic depression is a complex issue that requires the physician to
critically observe the patient and conduct appropriate follow-up following treatment to avoid severe adverse
events or relapse.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

REFERENCES
1. Rothschild AJ. Challenges in the treatment of
major depressive disorder with psychotic
features. Schizophr Bull. 2013;39(4):787-796.
doi:10.1093/schbul/sbt046
2. Gournellis R, Tournikioti K, Touloumi G, et al.
Psychotic (delusional) depression and completed
suicide: a systematic review and meta-
analysis. Ann Gen Psychiatry. 2018;17:39-51
doi:10.1186/s12991-018-0207-1
3. Farahani A, Correll CU. Are antipsychotics or
antidepressants needed for psychotic depression? a
systematic review and meta-analysis of trials
comparing antidepressant or antipsychotic
monotherapy with combination treatment. J Clin
Psychiatry. 2012;73(4):486-496.
doi:10.4088/JCP.11r07324
4. Wijkstra J, Lijmer J, Burger H, Cipriani A, Geddes
J, Nolen WA. Pharmacological treatment for
psychotic depression. Cochrane Database Syst
Rev. 2015;30(7):CD004044.
doi:10.1002/14651858.CD004044.pub4
5 Deshauer D, Moher D, Fergusson D, Moher E,
Sampson M, Grimshaw J. Selective serotonin reuptake
inhibitors for unipolar depression: a systematic review
of classic long-term randomized controlled trials.
CMAJ. 2008;178(10): 1293-1301.
doi:10.1503/cmaj.071068
6 Meyers BS, Flint AJ, Rothschild AJ, et al. ,
STOP-PD Group . A double-blind randomized
controlled trial of olanzapine plus sertraline vs
olanzapine plus placebo for psychotic depression:
the study of pharmacotherapy of psychotic
depression (STOP-PD). Arch Gen Psychiatry.
2009;66(8):838-847.
doi:10.1001/archgenpsychiatry.2009.79
7 Meyers BS, English J, Gabriele M, et al. ; STOP-
PD Study Group . A delusion assessment scale for
psychotic major depression: reliability, validity,
and utility. Biol Psychiatry. 2006;60(12):1336-
1342. doi:10.1016/j.biopsych.2006.05.033
8 Simpson GM, Angus JW. A rating scale for
extrapyramidal side effects. Acta Psychiatr Scand
Suppl. 1970;212(suppl 212):11-19.
doi:10.1111/j.1600-0447.1970.tb02066.x
1. Rothschild AJ. Challenges in the treatment of
major depressive disorder with psychotic
features. Schizophr Bull. 2013;39(4):787-796.
doi:10.1093/schbul/sbt046
2. Gournellis R, Tournikioti K, Touloumi G, et al.
Psychotic (delusional) depression and completed
suicide: a systematic review and meta-
analysis. Ann Gen Psychiatry. 2018;17:39-51
doi:10.1186/s12991-018-0207-1
3. Farahani A, Correll CU. Are antipsychotics or
antidepressants needed for psychotic depression? a
systematic review and meta-analysis of trials
comparing antidepressant or antipsychotic
monotherapy with combination treatment. J Clin
Psychiatry. 2012;73(4):486-496.
doi:10.4088/JCP.11r07324
4. Wijkstra J, Lijmer J, Burger H, Cipriani A, Geddes
J, Nolen WA. Pharmacological treatment for
psychotic depression. Cochrane Database Syst
Rev. 2015;30(7):CD004044.
doi:10.1002/14651858.CD004044.pub4
5 Deshauer D, Moher D, Fergusson D, Moher E,
Sampson M, Grimshaw J. Selective serotonin reuptake
inhibitors for unipolar depression: a systematic review
of classic long-term randomized controlled trials.
CMAJ. 2008;178(10): 1293-1301.
doi:10.1503/cmaj.071068
6 Meyers BS, Flint AJ, Rothschild AJ, et al. ,
STOP-PD Group . A double-blind randomized
controlled trial of olanzapine plus sertraline vs
olanzapine plus placebo for psychotic depression:
the study of pharmacotherapy of psychotic
depression (STOP-PD). Arch Gen Psychiatry.
2009;66(8):838-847.
doi:10.1001/archgenpsychiatry.2009.79
7 Meyers BS, English J, Gabriele M, et al. ; STOP-
PD Study Group . A delusion assessment scale for
psychotic major depression: reliability, validity,
and utility. Biol Psychiatry. 2006;60(12):1336-
1342. doi:10.1016/j.biopsych.2006.05.033
8 Simpson GM, Angus JW. A rating scale for
extrapyramidal side effects. Acta Psychiatr Scand
Suppl. 1970;212(suppl 212):11-19.
doi:10.1111/j.1600-0447.1970.tb02066.x
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.