Effectiveness of Oral Health Training for Type 1 Diabetic Patients
VerifiedAdded on 2022/09/02
|6
|1397
|25
Project
AI Summary
This project presents a comprehensive participant information sheet and consent form for a research study conducted at the University of Manchester, investigating the impact of verbal and nonverbal oral health training on individuals with Type 1 diabetes. The study aims to assess the effectiveness of different awareness methods on improving oral health in this specific patient population. Participants are recruited through the National Diabetes Services Scheme (NDSS) register and are informed about the study's purpose, procedures, potential benefits, and voluntary nature of participation. The research involves an initial dental examination, participation in different dental health wellness programs, and a final dental health assessment with a face-to-face interview. The study ensures confidentiality and ethical considerations, including data storage, handling, and participant rights, as well as the provisions for indemnity and ethical reviews. The results of the study will be published in a peer-reviewed journal, and participants will have access to the findings. The document also includes details on how the study addresses ethical concerns, obtains informed consent, and manages participant data. The project underscores the importance of informed consent, confidentiality, and the ethical conduct of medical research.

Running head: ETHICS
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1ETHICS
Participant Information Sheet
Version: 1.2
Impact of verbal and nonverbal oral health training on improving the Type 1 diabetic
patient’s oral health
Invitation
You are requested to take a part in this study which will include various awareness programs
and an oral health assessment program. Hope this study will bring benefits for you in terms of
health and benefits for us in terms of knowledge. Kindly go through the following
information details regarding this study.
Purpose of the study
The purpose of this study is to find if different awareness methods have different impact on
oral health within the people who are suffering from the diabetes type 1. The aim is to assess
the effectiveness of verbal and non-verbal oral health training in Type 1 Diabetic patients.
Why have I been chosen?
This study is aimed to find the effectiveness of verbal and non-verbal oral health training
program on people with type 1 diabetes. The participants have been selected through National
Diabetes Services Scheme (NDSS) register. Your medical details related to diabetic issues
have been found in the NDSS register and therefore, you have been selected.
Do I have to take part?
You participation is voluntary not mandatory. You will not have any strict obligation to
continue with this study. You can leave the research procedures anytime you want.
What do I have to do?
You have to go through an initial dental examination for all participants. After that the
participants will be included in different dental health wellness program. Finally, again the
dental health assessment process will be executed along with a face to face interview.
What is the drug, device or procedure being tested?
This study will not include any drug or device based intervention. You have been assessed
with some medical tools where medical professional will be asking you some questions about
your oral health and inspect your dental condition visually. The tools are Beck Oral
Assessment Scale (BOAS), mucosal-plaque score (MPS), and the Oral Health Assessment.
All these tools are medically approved and highly reliable.
Participant Information Sheet
Version: 1.2
Impact of verbal and nonverbal oral health training on improving the Type 1 diabetic
patient’s oral health
Invitation
You are requested to take a part in this study which will include various awareness programs
and an oral health assessment program. Hope this study will bring benefits for you in terms of
health and benefits for us in terms of knowledge. Kindly go through the following
information details regarding this study.
Purpose of the study
The purpose of this study is to find if different awareness methods have different impact on
oral health within the people who are suffering from the diabetes type 1. The aim is to assess
the effectiveness of verbal and non-verbal oral health training in Type 1 Diabetic patients.
Why have I been chosen?
This study is aimed to find the effectiveness of verbal and non-verbal oral health training
program on people with type 1 diabetes. The participants have been selected through National
Diabetes Services Scheme (NDSS) register. Your medical details related to diabetic issues
have been found in the NDSS register and therefore, you have been selected.
Do I have to take part?
You participation is voluntary not mandatory. You will not have any strict obligation to
continue with this study. You can leave the research procedures anytime you want.
What do I have to do?
You have to go through an initial dental examination for all participants. After that the
participants will be included in different dental health wellness program. Finally, again the
dental health assessment process will be executed along with a face to face interview.
What is the drug, device or procedure being tested?
This study will not include any drug or device based intervention. You have been assessed
with some medical tools where medical professional will be asking you some questions about
your oral health and inspect your dental condition visually. The tools are Beck Oral
Assessment Scale (BOAS), mucosal-plaque score (MPS), and the Oral Health Assessment.
All these tools are medically approved and highly reliable.

2ETHICS
What are the alternatives available?
There are two alternatives in this pregame. Either you will be provided with written
instruction about how to maintain a good oral health, or you will be provided with verbal and
non-verbal oral health training audio-visual presentation and interpersonal communication
What are the potential side effects or risks in taking part?
Since, this study entirely is based on non-medical intervention, it will not cause any side
effect or risk to the participants because of their participation. You just need to take part in
this project for particular duration.
What are the possible benefits of taking part?
Instead of having any risk, participants of this study will have a free oral health check-up
using various tools. Apart from that you will be randomly selected in any of the two
participant groups. If you are selected in you will be also provided with oral health related
training which can have positive impact on your oral health in the long term.
Do our study team have the necessary skills/resources to conduct this research?
The study team includes Principal Investigator, Research Associate and Assistant Researcher.
All these team member have adequate resources and skills to conduct to study
What happens when the research stops?
When the research stops, you with other participants will be provided with a free copy of
training hand-out on oral health. After the study completes you will be communicated again
for once to know what would be the outcome of this study.
What do I do if there is a problem?
In case of any problem, like other participants you will be able to directly communicate with
our research associates and the principal investigator regarding any issues. You will be also
able to quit this study anytime you want.
Will the study team examine each stage of the process for ethical considerations?
The ethical considerations has been checked in four major steps namely, before the
intervention, after the intervention, before the assessment and after the assessment.
Will my taking part in this research be kept confidential?
What are the alternatives available?
There are two alternatives in this pregame. Either you will be provided with written
instruction about how to maintain a good oral health, or you will be provided with verbal and
non-verbal oral health training audio-visual presentation and interpersonal communication
What are the potential side effects or risks in taking part?
Since, this study entirely is based on non-medical intervention, it will not cause any side
effect or risk to the participants because of their participation. You just need to take part in
this project for particular duration.
What are the possible benefits of taking part?
Instead of having any risk, participants of this study will have a free oral health check-up
using various tools. Apart from that you will be randomly selected in any of the two
participant groups. If you are selected in you will be also provided with oral health related
training which can have positive impact on your oral health in the long term.
Do our study team have the necessary skills/resources to conduct this research?
The study team includes Principal Investigator, Research Associate and Assistant Researcher.
All these team member have adequate resources and skills to conduct to study
What happens when the research stops?
When the research stops, you with other participants will be provided with a free copy of
training hand-out on oral health. After the study completes you will be communicated again
for once to know what would be the outcome of this study.
What do I do if there is a problem?
In case of any problem, like other participants you will be able to directly communicate with
our research associates and the principal investigator regarding any issues. You will be also
able to quit this study anytime you want.
Will the study team examine each stage of the process for ethical considerations?
The ethical considerations has been checked in four major steps namely, before the
intervention, after the intervention, before the assessment and after the assessment.
Will my taking part in this research be kept confidential?
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3ETHICS
As per privacy and security concerns, participant's details will be strictly confidential,
guaranteed and will not be disclosed following the national code and data privacy and
security guidelines University of Manchester template on Confidentiality and ethical
consideration for accessing patient’s identifiable information as well as University code of
conduct.
How will data be recorded and stored?
Initially the data will be recorded in physical paper and pen. After that the data will be stored
in a secured digital database system for data storage and analysis. The data collected from the
participations will be stored in an encrypted digital database system which will be only
accessed by the research teams. It is assured that the raw data will not be used for any other
researches or any other purpose.
What happens if relevant new information becomes available?
In case, new information becomes available regarding the chosen topic of this study, the
study will still be continued as expected to testify the validity and reliability of the newly
available information.
What happens if I don’t want to continue on the study?
Like other participants you will be able to directly communicate with our research associates
and the principal investigators regarding your concern. You can discontinue this program
anytime you want.
Will any genetic tests be done?
This study will not conduct any genetic test
What will happen to the results of the study?
The final outcome of the study will be available in peered review journal of University Of
Manchester. You will be provided with the soft copy link through mail where you can see the
entire study after publication. The raw data you will provide will be kept in a secured and
private digital database system.
How will capacity to consent be assessed?
The capacity of consent will be assessed by ethical committee and the research coordination
board of the university
As per privacy and security concerns, participant's details will be strictly confidential,
guaranteed and will not be disclosed following the national code and data privacy and
security guidelines University of Manchester template on Confidentiality and ethical
consideration for accessing patient’s identifiable information as well as University code of
conduct.
How will data be recorded and stored?
Initially the data will be recorded in physical paper and pen. After that the data will be stored
in a secured digital database system for data storage and analysis. The data collected from the
participations will be stored in an encrypted digital database system which will be only
accessed by the research teams. It is assured that the raw data will not be used for any other
researches or any other purpose.
What happens if relevant new information becomes available?
In case, new information becomes available regarding the chosen topic of this study, the
study will still be continued as expected to testify the validity and reliability of the newly
available information.
What happens if I don’t want to continue on the study?
Like other participants you will be able to directly communicate with our research associates
and the principal investigators regarding your concern. You can discontinue this program
anytime you want.
Will any genetic tests be done?
This study will not conduct any genetic test
What will happen to the results of the study?
The final outcome of the study will be available in peered review journal of University Of
Manchester. You will be provided with the soft copy link through mail where you can see the
entire study after publication. The raw data you will provide will be kept in a secured and
private digital database system.
How will capacity to consent be assessed?
The capacity of consent will be assessed by ethical committee and the research coordination
board of the university
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4ETHICS
What will be done and have been done regarding consent?
The consent from will be provided to the potential participants with the participation request
letter, participants information sheet and through face to face communication. The consent
forms will be provided to the research assistant and he will be responsible to collect the
consent from the potential research participants.
Who is organizing and funding the research?
The major funding resources are Personal Grants and Grants from University of Manchester
What are the provisions for indemnity?
The obligation to pay for any loss or damage goes to the principal researcher or the research
coordinator.
Who has reviewed the research proposal?
This research proposal has been reviewed by the ethical committee and the research
coordination board of the University <Name of the University>
Contact details for further information?
What will be done and have been done regarding consent?
The consent from will be provided to the potential participants with the participation request
letter, participants information sheet and through face to face communication. The consent
forms will be provided to the research assistant and he will be responsible to collect the
consent from the potential research participants.
Who is organizing and funding the research?
The major funding resources are Personal Grants and Grants from University of Manchester
What are the provisions for indemnity?
The obligation to pay for any loss or damage goes to the principal researcher or the research
coordinator.
Who has reviewed the research proposal?
This research proposal has been reviewed by the ethical committee and the research
coordination board of the University <Name of the University>
Contact details for further information?
5ETHICS
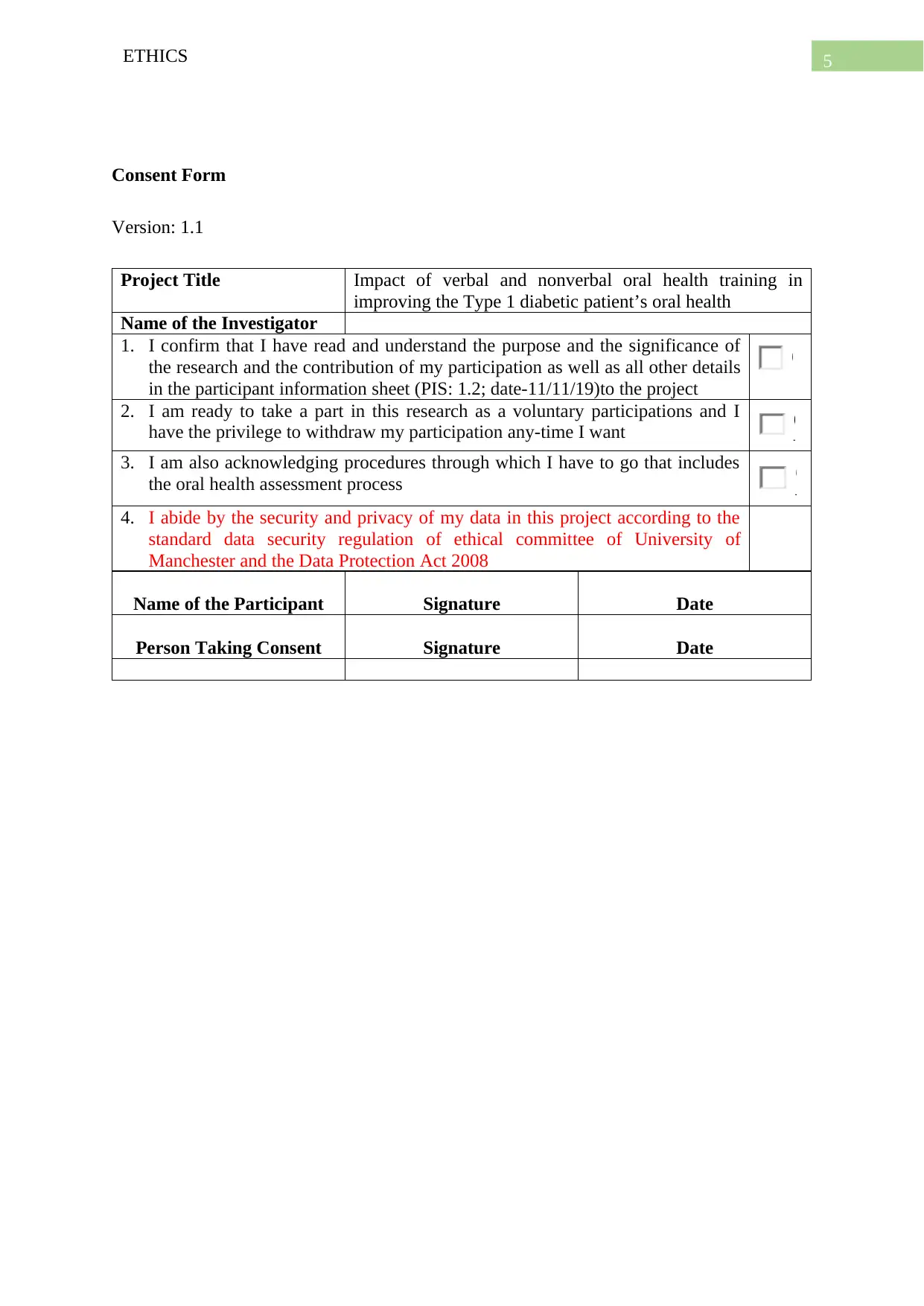
Consent Form
Version: 1.1
Project Title Impact of verbal and nonverbal oral health training in
improving the Type 1 diabetic patient’s oral health
Name of the Investigator
1. I confirm that I have read and understand the purpose and the significance of
the research and the contribution of my participation as well as all other details
in the participant information sheet (PIS: 1.2; date-11/11/19)to the project
2. I am ready to take a part in this research as a voluntary participations and I
have the privilege to withdraw my participation any-time I want
3. I am also acknowledging procedures through which I have to go that includes
the oral health assessment process
4. I abide by the security and privacy of my data in this project according to the
standard data security regulation of ethical committee of University of
Manchester and the Data Protection Act 2008
Name of the Participant Signature Date
Person Taking Consent Signature Date
C
h
C
h
C
h
Consent Form
Version: 1.1
Project Title Impact of verbal and nonverbal oral health training in
improving the Type 1 diabetic patient’s oral health
Name of the Investigator
1. I confirm that I have read and understand the purpose and the significance of
the research and the contribution of my participation as well as all other details
in the participant information sheet (PIS: 1.2; date-11/11/19)to the project
2. I am ready to take a part in this research as a voluntary participations and I
have the privilege to withdraw my participation any-time I want
3. I am also acknowledging procedures through which I have to go that includes
the oral health assessment process
4. I abide by the security and privacy of my data in this project according to the
standard data security regulation of ethical committee of University of
Manchester and the Data Protection Act 2008
Name of the Participant Signature Date
Person Taking Consent Signature Date
C
h
C
h
C
h
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




