Organic Chemistry Lab Report: Extraction, Distribution Coefficient
VerifiedAdded on 2020/04/21
|12
|1784
|587
Report
AI Summary
This organic chemistry lab report details experiments on extraction techniques and the determination of distribution coefficients. The report covers the extraction of water-soluble dyes (methylene blue and methylene red) using ether and water, observing the distribution of dyes between the two solvents. It also examines the salting-out effect using crystal violet and NaCl. Furthermore, the report includes an experiment to determine the distribution coefficient of adipic acid between water and diethyl ether, involving titration with NaOH. Observations, tables of reagents, and results, including calculations of the distribution coefficient, are presented. The discussion analyzes the experimental results, explaining the principles of extraction, the impact of salting out, and the effectiveness of multiple extractions. The report concludes with a summary of the findings and answers to relevant questions, supported by cited works.

Surname1
LABARATORY REPORT IN ORGANIC CHEMISTRY
Student’s Name
Professor’s Name
Course
Course Number
Date
Group Day
LABARATORY REPORT IN ORGANIC CHEMISTRY
Student’s Name
Professor’s Name
Course
Course Number
Date
Group Day
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Surname2
Organic Chemistry Lab Report
Introduction
Organic chemistry is a field of science that deals with the study of compounds containing
carbon atoms. Mostly the most common compounds that are given a lot of consideration are
hydrocarbons which are made only of hydrogen and carbon (Jonathan Clayden). In the study of
organic chemistry both the physical and chemical properties of these organic compounds are
analyzed as well as understanding how these components are chemically constituted.
Purpose
The purpose of this lab experiment is to equip the student with extraction process skills
needed in separating an organic material from an aqueous solution and being able to determine
the distribution coefficient.
Materials and Methods
Test tubes
125ml Erlenmeyer flask
Separatory flask
Beaker
Extraction of Water Soluble Dyes
1 ml of ether, 1 ml of distilled water and 1 drop of 0.006 M solution of methylene blue
were placed in a test tube and the shaken thoroughly for one minute to mix the contents. The
process was repeated by using 1 drop of 0.006 M solution of methylene red. The contents of the
two test tubes were poured together and the resulting mixture shaken for one minute.
Organic Chemistry Lab Report
Introduction
Organic chemistry is a field of science that deals with the study of compounds containing
carbon atoms. Mostly the most common compounds that are given a lot of consideration are
hydrocarbons which are made only of hydrogen and carbon (Jonathan Clayden). In the study of
organic chemistry both the physical and chemical properties of these organic compounds are
analyzed as well as understanding how these components are chemically constituted.
Purpose
The purpose of this lab experiment is to equip the student with extraction process skills
needed in separating an organic material from an aqueous solution and being able to determine
the distribution coefficient.
Materials and Methods
Test tubes
125ml Erlenmeyer flask
Separatory flask
Beaker
Extraction of Water Soluble Dyes
1 ml of ether, 1 ml of distilled water and 1 drop of 0.006 M solution of methylene blue
were placed in a test tube and the shaken thoroughly for one minute to mix the contents. The
process was repeated by using 1 drop of 0.006 M solution of methylene red. The contents of the
two test tubes were poured together and the resulting mixture shaken for one minute.

Surname3
Salting out Effect in Extractions
5 ml of distilled water, 1 drop of 0.003 M aqueous crystal violet and 0.5 ml of 1-butanol
were placed in two different test tubes and shaken until the color of the crystal violet had been
evenly distributed in the two layers. Solid NaCl was added to one test tube up to saturation and
both tubes shaken for one minute. Amount of crystal violet in the aqueous layers of the two
samples was compared
Determining the Distribution Coefficient
35 ml of 1% aqueous adipic acid was added into a dry covered beaker and 10ml of the
solution pipetted into a 125ml Erlenmeyer flask and the adipic acid titrated using 0.05 M NaOH
in the presence of phenolphthalein indicator.
A second 10ml adipic acid sample was titrated into a separatory funnel and 10ml of
diethyl ether added before the separatory funnel was shaken once and then immediately vented.
The separatory funnel was then shaken for two minutes with the aim of extracting the adipic acid
into the ether layer (Laboratory Manual). The aqueous layers was left to separate after which it
was transferred to a clean 125ml Erlenmeyer flask. A drop of phenolphthalein indicator was
added into the flask and the aqueous layer titrated using 0.05 M of NaOH.
The separatory funnel was cleaned and 10ml of adipic acid pipetted into it. 10ml of ether
was added and the separatory funnel shaken. The ether layer was given time to separate and then
discarded. The remaining aqueous layer was again extracted using 10ml of ether after which it
was transferred to a clean 125ml Erlenmeyer flask and adipic acid titrated with 0.5 M NaOH
solution to phenolphthalein endpoint.
Table of Reagents and Products
Salting out Effect in Extractions
5 ml of distilled water, 1 drop of 0.003 M aqueous crystal violet and 0.5 ml of 1-butanol
were placed in two different test tubes and shaken until the color of the crystal violet had been
evenly distributed in the two layers. Solid NaCl was added to one test tube up to saturation and
both tubes shaken for one minute. Amount of crystal violet in the aqueous layers of the two
samples was compared
Determining the Distribution Coefficient
35 ml of 1% aqueous adipic acid was added into a dry covered beaker and 10ml of the
solution pipetted into a 125ml Erlenmeyer flask and the adipic acid titrated using 0.05 M NaOH
in the presence of phenolphthalein indicator.
A second 10ml adipic acid sample was titrated into a separatory funnel and 10ml of
diethyl ether added before the separatory funnel was shaken once and then immediately vented.
The separatory funnel was then shaken for two minutes with the aim of extracting the adipic acid
into the ether layer (Laboratory Manual). The aqueous layers was left to separate after which it
was transferred to a clean 125ml Erlenmeyer flask. A drop of phenolphthalein indicator was
added into the flask and the aqueous layer titrated using 0.05 M of NaOH.
The separatory funnel was cleaned and 10ml of adipic acid pipetted into it. 10ml of ether
was added and the separatory funnel shaken. The ether layer was given time to separate and then
discarded. The remaining aqueous layer was again extracted using 10ml of ether after which it
was transferred to a clean 125ml Erlenmeyer flask and adipic acid titrated with 0.5 M NaOH
solution to phenolphthalein endpoint.
Table of Reagents and Products
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
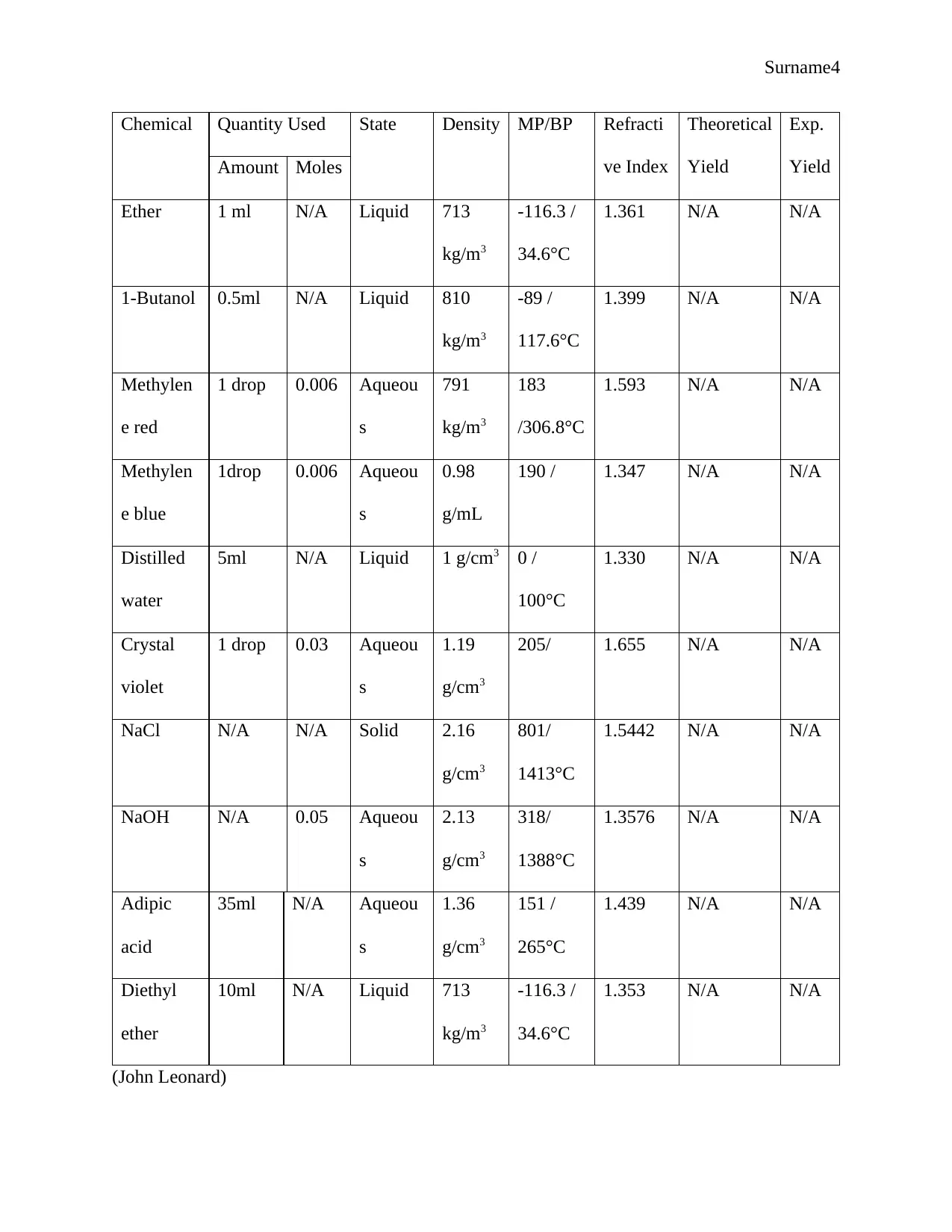
Surname4
Chemical Quantity Used State Density MP/BP Refracti
ve Index
Theoretical
Yield
Exp.
YieldAmount Moles
Ether 1 ml N/A Liquid 713
kg/m3
-116.3 /
34.6°C
1.361 N/A N/A
1-Butanol 0.5ml N/A Liquid 810
kg/m3
-89 /
117.6°C
1.399 N/A N/A
Methylen
e red
1 drop 0.006 Aqueou
s
791
kg/m3
183
/306.8°C
1.593 N/A N/A
Methylen
e blue
1drop 0.006 Aqueou
s
0.98
g/mL
190 / 1.347 N/A N/A
Distilled
water
5ml N/A Liquid 1 g/cm3 0 /
100°C
1.330 N/A N/A
Crystal
violet
1 drop 0.03 Aqueou
s
1.19
g/cm3
205/ 1.655 N/A N/A
NaCl N/A N/A Solid 2.16
g/cm3
801/
1413°C
1.5442 N/A N/A
NaOH N/A 0.05 Aqueou
s
2.13
g/cm3
318/
1388°C
1.3576 N/A N/A
Adipic
acid
35ml N/A Aqueou
s
1.36
g/cm3
151 /
265°C
1.439 N/A N/A
Diethyl
ether
10ml N/A Liquid 713
kg/m3
-116.3 /
34.6°C
1.353 N/A N/A
(John Leonard)
Chemical Quantity Used State Density MP/BP Refracti
ve Index
Theoretical
Yield
Exp.
YieldAmount Moles
Ether 1 ml N/A Liquid 713
kg/m3
-116.3 /
34.6°C
1.361 N/A N/A
1-Butanol 0.5ml N/A Liquid 810
kg/m3
-89 /
117.6°C
1.399 N/A N/A
Methylen
e red
1 drop 0.006 Aqueou
s
791
kg/m3
183
/306.8°C
1.593 N/A N/A
Methylen
e blue
1drop 0.006 Aqueou
s
0.98
g/mL
190 / 1.347 N/A N/A
Distilled
water
5ml N/A Liquid 1 g/cm3 0 /
100°C
1.330 N/A N/A
Crystal
violet
1 drop 0.03 Aqueou
s
1.19
g/cm3
205/ 1.655 N/A N/A
NaCl N/A N/A Solid 2.16
g/cm3
801/
1413°C
1.5442 N/A N/A
NaOH N/A 0.05 Aqueou
s
2.13
g/cm3
318/
1388°C
1.3576 N/A N/A
Adipic
acid
35ml N/A Aqueou
s
1.36
g/cm3
151 /
265°C
1.439 N/A N/A
Diethyl
ether
10ml N/A Liquid 713
kg/m3
-116.3 /
34.6°C
1.353 N/A N/A
(John Leonard)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
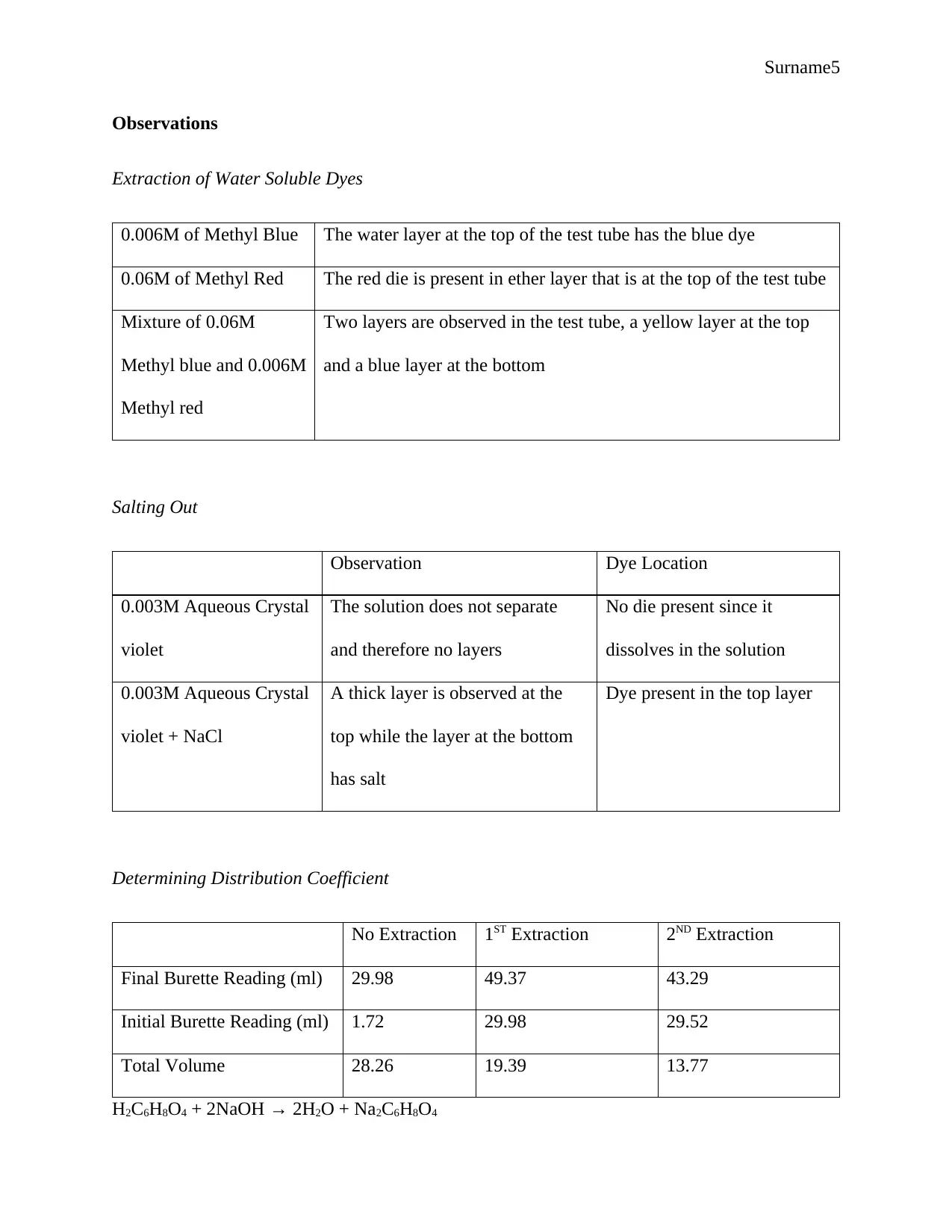
Surname5
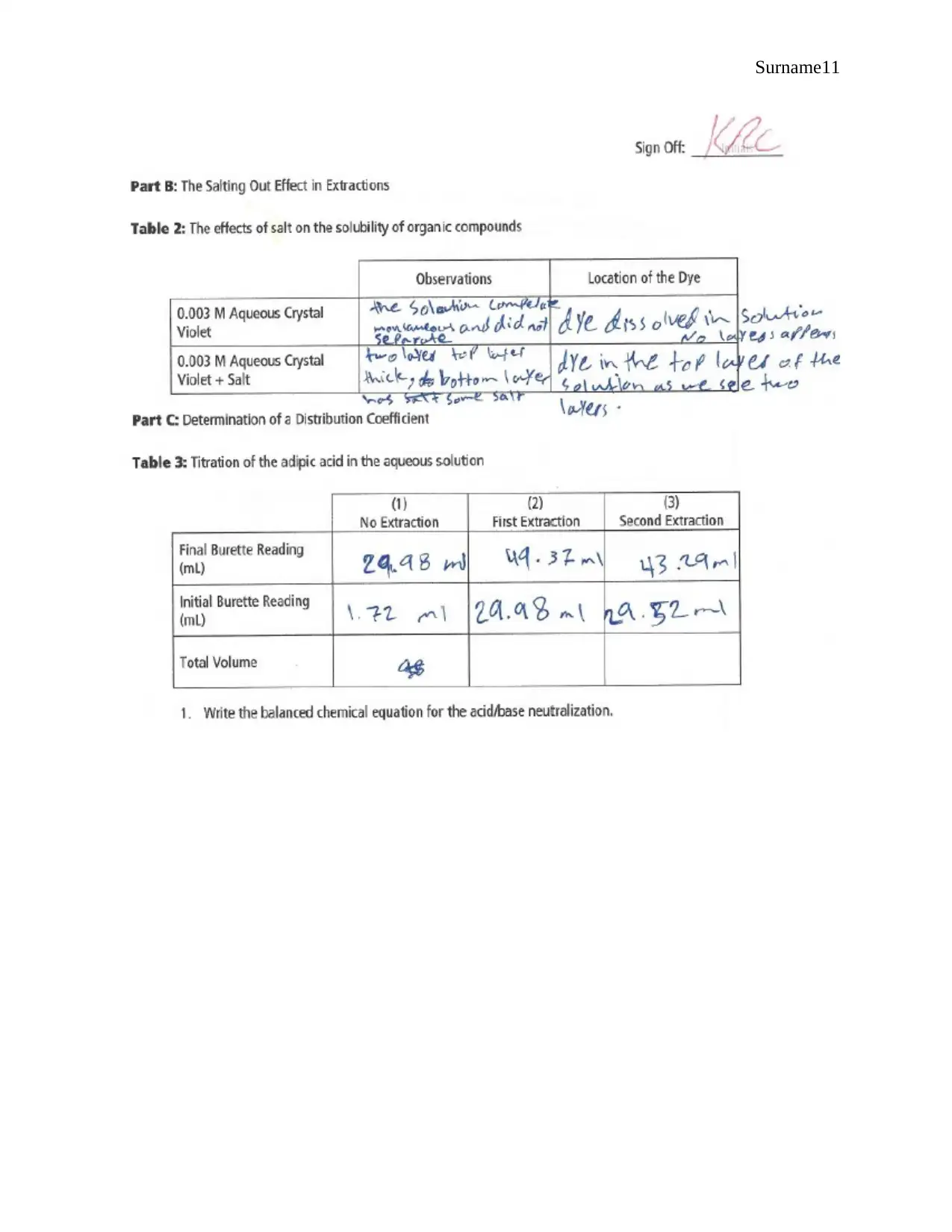
Observations
Extraction of Water Soluble Dyes
0.006M of Methyl Blue The water layer at the top of the test tube has the blue dye
0.06M of Methyl Red The red die is present in ether layer that is at the top of the test tube
Mixture of 0.06M
Methyl blue and 0.006M
Methyl red
Two layers are observed in the test tube, a yellow layer at the top
and a blue layer at the bottom
Salting Out
Observation Dye Location
0.003M Aqueous Crystal
violet
The solution does not separate
and therefore no layers
No die present since it
dissolves in the solution
0.003M Aqueous Crystal
violet + NaCl
A thick layer is observed at the
top while the layer at the bottom
has salt
Dye present in the top layer
Determining Distribution Coefficient
No Extraction 1ST Extraction 2ND Extraction
Final Burette Reading (ml) 29.98 49.37 43.29
Initial Burette Reading (ml) 1.72 29.98 29.52
Total Volume 28.26 19.39 13.77
H2C6H8O4 + 2NaOH → 2H2O + Na2C6H8O4
Observations
Extraction of Water Soluble Dyes
0.006M of Methyl Blue The water layer at the top of the test tube has the blue dye
0.06M of Methyl Red The red die is present in ether layer that is at the top of the test tube
Mixture of 0.06M
Methyl blue and 0.006M
Methyl red
Two layers are observed in the test tube, a yellow layer at the top
and a blue layer at the bottom
Salting Out
Observation Dye Location
0.003M Aqueous Crystal
violet
The solution does not separate
and therefore no layers
No die present since it
dissolves in the solution
0.003M Aqueous Crystal
violet + NaCl
A thick layer is observed at the
top while the layer at the bottom
has salt
Dye present in the top layer
Determining Distribution Coefficient
No Extraction 1ST Extraction 2ND Extraction
Final Burette Reading (ml) 29.98 49.37 43.29
Initial Burette Reading (ml) 1.72 29.98 29.52
Total Volume 28.26 19.39 13.77
H2C6H8O4 + 2NaOH → 2H2O + Na2C6H8O4
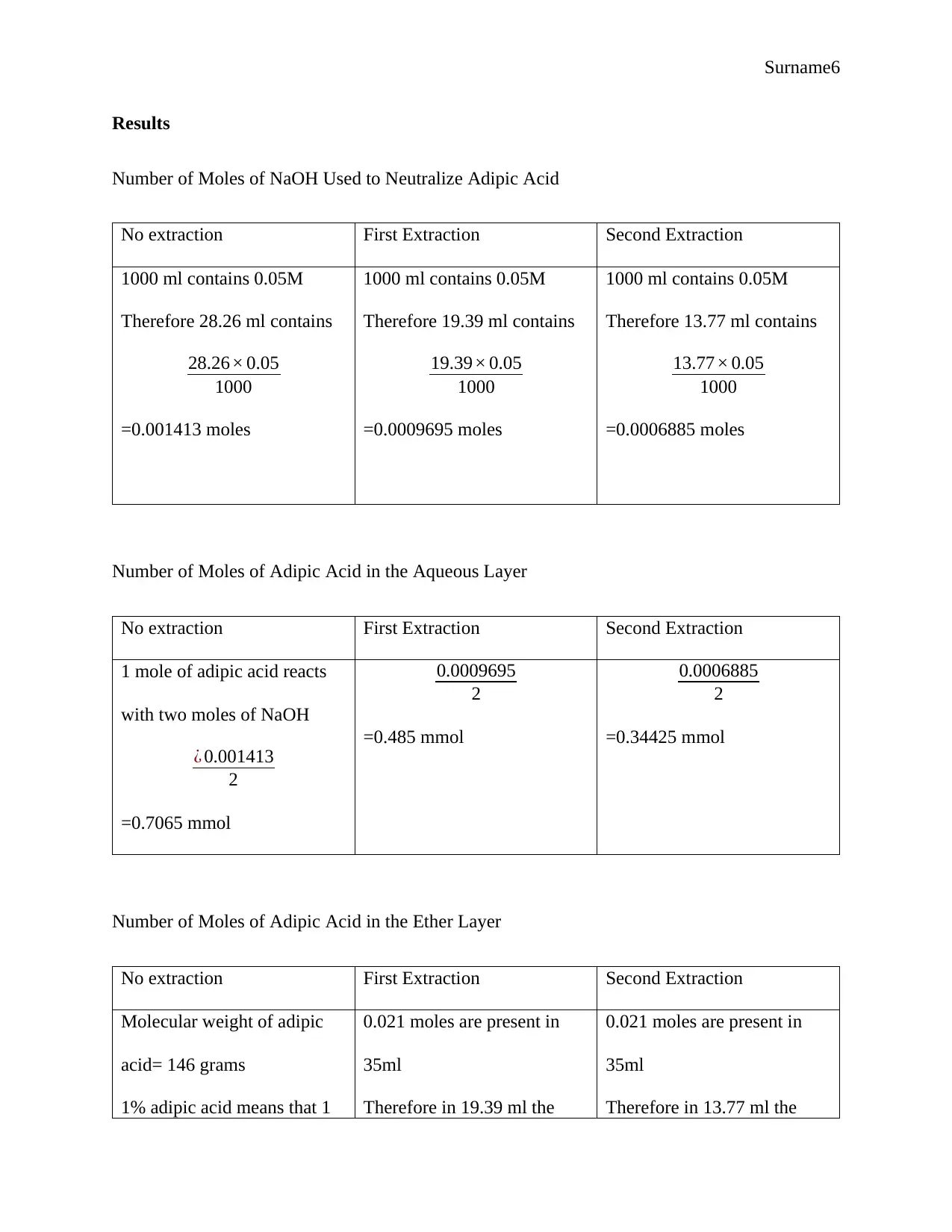
Surname6
Results
Number of Moles of NaOH Used to Neutralize Adipic Acid
No extraction First Extraction Second Extraction
1000 ml contains 0.05M
Therefore 28.26 ml contains
28.26× 0.05
1000
=0.001413 moles
1000 ml contains 0.05M
Therefore 19.39 ml contains
19.39× 0.05
1000
=0.0009695 moles
1000 ml contains 0.05M
Therefore 13.77 ml contains
13.77× 0.05
1000
=0.0006885 moles
Number of Moles of Adipic Acid in the Aqueous Layer
No extraction First Extraction Second Extraction
1 mole of adipic acid reacts
with two moles of NaOH
¿ 0.001413
2
=0.7065 mmol
0.0009695
2
=0.485 mmol
0.0006885
2
=0.34425 mmol
Number of Moles of Adipic Acid in the Ether Layer
No extraction First Extraction Second Extraction
Molecular weight of adipic
acid= 146 grams
1% adipic acid means that 1
0.021 moles are present in
35ml
Therefore in 19.39 ml the
0.021 moles are present in
35ml
Therefore in 13.77 ml the
Results
Number of Moles of NaOH Used to Neutralize Adipic Acid
No extraction First Extraction Second Extraction
1000 ml contains 0.05M
Therefore 28.26 ml contains
28.26× 0.05
1000
=0.001413 moles
1000 ml contains 0.05M
Therefore 19.39 ml contains
19.39× 0.05
1000
=0.0009695 moles
1000 ml contains 0.05M
Therefore 13.77 ml contains
13.77× 0.05
1000
=0.0006885 moles
Number of Moles of Adipic Acid in the Aqueous Layer
No extraction First Extraction Second Extraction
1 mole of adipic acid reacts
with two moles of NaOH
¿ 0.001413
2
=0.7065 mmol
0.0009695
2
=0.485 mmol
0.0006885
2
=0.34425 mmol
Number of Moles of Adipic Acid in the Ether Layer
No extraction First Extraction Second Extraction
Molecular weight of adipic
acid= 146 grams
1% adipic acid means that 1
0.021 moles are present in
35ml
Therefore in 19.39 ml the
0.021 moles are present in
35ml
Therefore in 13.77 ml the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
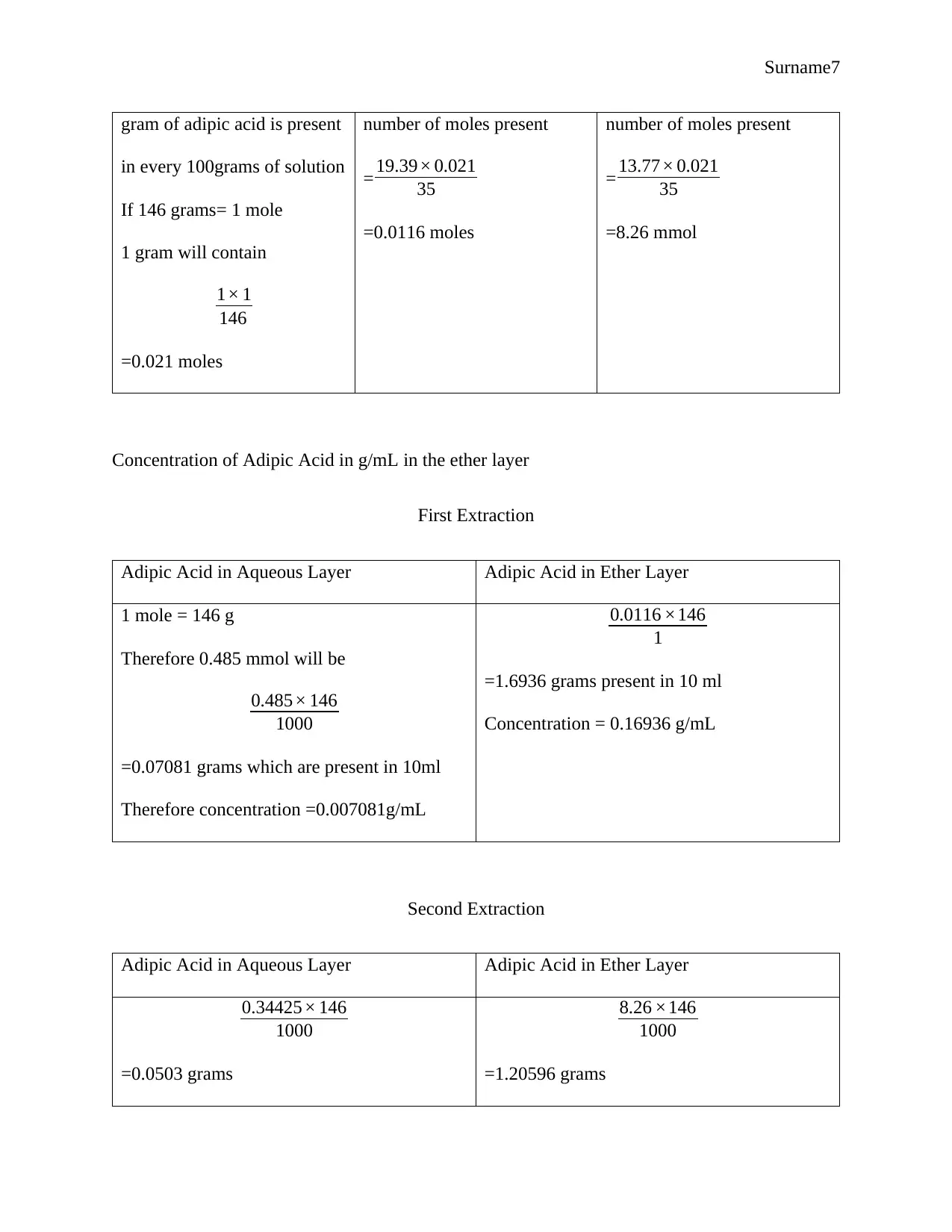
Surname7
gram of adipic acid is present
in every 100grams of solution
If 146 grams= 1 mole
1 gram will contain
1× 1
146
=0.021 moles
number of moles present
= 19.39× 0.021
35
=0.0116 moles
number of moles present
= 13.77× 0.021
35
=8.26 mmol
Concentration of Adipic Acid in g/mL in the ether layer
First Extraction
Adipic Acid in Aqueous Layer Adipic Acid in Ether Layer
1 mole = 146 g
Therefore 0.485 mmol will be
0.485× 146
1000
=0.07081 grams which are present in 10ml
Therefore concentration =0.007081g/mL
0.0116 ×146
1
=1.6936 grams present in 10 ml
Concentration = 0.16936 g/mL
Second Extraction
Adipic Acid in Aqueous Layer Adipic Acid in Ether Layer
0.34425× 146
1000
=0.0503 grams
8.26 ×146
1000
=1.20596 grams
gram of adipic acid is present
in every 100grams of solution
If 146 grams= 1 mole
1 gram will contain
1× 1
146
=0.021 moles
number of moles present
= 19.39× 0.021
35
=0.0116 moles
number of moles present
= 13.77× 0.021
35
=8.26 mmol
Concentration of Adipic Acid in g/mL in the ether layer
First Extraction
Adipic Acid in Aqueous Layer Adipic Acid in Ether Layer
1 mole = 146 g
Therefore 0.485 mmol will be
0.485× 146
1000
=0.07081 grams which are present in 10ml
Therefore concentration =0.007081g/mL
0.0116 ×146
1
=1.6936 grams present in 10 ml
Concentration = 0.16936 g/mL
Second Extraction
Adipic Acid in Aqueous Layer Adipic Acid in Ether Layer
0.34425× 146
1000
=0.0503 grams
8.26 ×146
1000
=1.20596 grams
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Surname8
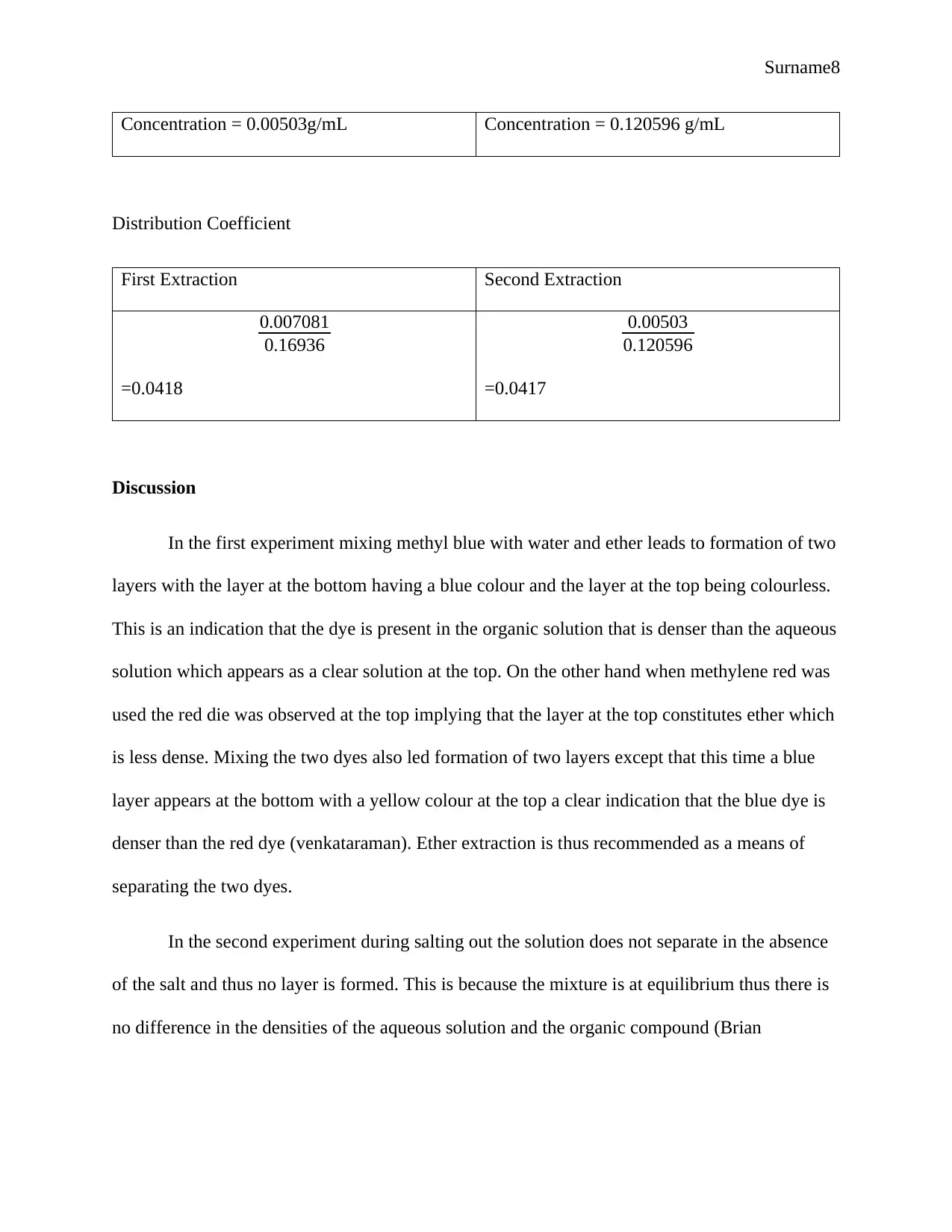
Concentration = 0.00503g/mL Concentration = 0.120596 g/mL
Distribution Coefficient
First Extraction Second Extraction
0.007081
0.16936
=0.0418
0.00503
0.120596
=0.0417
Discussion
In the first experiment mixing methyl blue with water and ether leads to formation of two
layers with the layer at the bottom having a blue colour and the layer at the top being colourless.
This is an indication that the dye is present in the organic solution that is denser than the aqueous
solution which appears as a clear solution at the top. On the other hand when methylene red was
used the red die was observed at the top implying that the layer at the top constitutes ether which
is less dense. Mixing the two dyes also led formation of two layers except that this time a blue
layer appears at the bottom with a yellow colour at the top a clear indication that the blue dye is
denser than the red dye (venkataraman). Ether extraction is thus recommended as a means of
separating the two dyes.
In the second experiment during salting out the solution does not separate in the absence
of the salt and thus no layer is formed. This is because the mixture is at equilibrium thus there is
no difference in the densities of the aqueous solution and the organic compound (Brian
Concentration = 0.00503g/mL Concentration = 0.120596 g/mL
Distribution Coefficient
First Extraction Second Extraction
0.007081
0.16936
=0.0418
0.00503
0.120596
=0.0417
Discussion
In the first experiment mixing methyl blue with water and ether leads to formation of two
layers with the layer at the bottom having a blue colour and the layer at the top being colourless.
This is an indication that the dye is present in the organic solution that is denser than the aqueous
solution which appears as a clear solution at the top. On the other hand when methylene red was
used the red die was observed at the top implying that the layer at the top constitutes ether which
is less dense. Mixing the two dyes also led formation of two layers except that this time a blue
layer appears at the bottom with a yellow colour at the top a clear indication that the blue dye is
denser than the red dye (venkataraman). Ether extraction is thus recommended as a means of
separating the two dyes.
In the second experiment during salting out the solution does not separate in the absence
of the salt and thus no layer is formed. This is because the mixture is at equilibrium thus there is
no difference in the densities of the aqueous solution and the organic compound (Brian

Surname9
Berkowitz). Addition of NaCl to the mixture leads to formation of two layers because the salt
makes the aqueous layer denser than the organic layer.
In the third experiment it is clear that the distribution coefficients are calculated to be
0.418 and 0.417 which are almost similar thus it is more effective to extract ether two times in
small volumes (Sirkar).
Conclusion
Extraction of ether is a very reliable and efficient way of separating two water soluble
dyes. Ether is an organic compound with a density of 0.7 g/cm3 thus it is less dense than water
and will appear as the top layer (Tmh). Salting out aids in recovering organic compounds that are
extremely soluble in water. The mixture of alcohol in the case above being 1-butanol, aqueous
crystal violet, water and salt results in the formation of two immiscible liquids (Cooper). The
reaction between adipic acid and NaOH is a neutralization reaction leading in the formation of a
salt and water.
Questions
1) An aqueous mixture of methyl blue and methyl red can be separated by addition of ether.
Since ether is less dense it settles at the top mixing with the red dye and the blue dye
being denser settles at the bottom. Ether is then extracted from the mixture and the red
dye retrieved.
2) This can be done by addition of a salt and an alcohol to the aqueous solution. This leads
two formation of two immiscible layers of the organic compound and the solution
Berkowitz). Addition of NaCl to the mixture leads to formation of two layers because the salt
makes the aqueous layer denser than the organic layer.
In the third experiment it is clear that the distribution coefficients are calculated to be
0.418 and 0.417 which are almost similar thus it is more effective to extract ether two times in
small volumes (Sirkar).
Conclusion
Extraction of ether is a very reliable and efficient way of separating two water soluble
dyes. Ether is an organic compound with a density of 0.7 g/cm3 thus it is less dense than water
and will appear as the top layer (Tmh). Salting out aids in recovering organic compounds that are
extremely soluble in water. The mixture of alcohol in the case above being 1-butanol, aqueous
crystal violet, water and salt results in the formation of two immiscible liquids (Cooper). The
reaction between adipic acid and NaOH is a neutralization reaction leading in the formation of a
salt and water.
Questions
1) An aqueous mixture of methyl blue and methyl red can be separated by addition of ether.
Since ether is less dense it settles at the top mixing with the red dye and the blue dye
being denser settles at the bottom. Ether is then extracted from the mixture and the red
dye retrieved.
2) This can be done by addition of a salt and an alcohol to the aqueous solution. This leads
two formation of two immiscible layers of the organic compound and the solution
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Surname10
3) Addition of NaCl to a mixture of water, ether and methyl blue increases the amount of
methyl blue in the aqueous region because the salt increases the density of the aqueous
solution.
4) The value of K is small for both methylene blue and methylene red because C is the
concentration in grams/ml and since ethyl is lighter that water of the same volume the
value of K is smaller.
3) Addition of NaCl to a mixture of water, ether and methyl blue increases the amount of
methyl blue in the aqueous region because the salt increases the density of the aqueous
solution.
4) The value of K is small for both methylene blue and methylene red because C is the
concentration in grams/ml and since ethyl is lighter that water of the same volume the
value of K is smaller.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Surname11

Surname12
Works Cited
Brian Berkowitz, Ishal Dror, Bruno Yaron. Contaminant Geochemistry. Los Angeles: Springer
Science & Business, 2014. Book.
Cooper, Alan. Biophysical Chemistry. Manchester: Royal Society of Chemistry, 2011. Book.
John Leonard, Barry Lygo, Garry Procter. Advanced Practical Organic Chemistry. New York:
CRC Press, 2013. Book.
Jonathan Clayden, Nick Greeves, Stuart Warren. Organic Chemistry. New York: OUP Oxford,
2012. Book.
Laboratory Manual
Sirkar, Kamalesh. Separation of Molecules, Macromolecules and Particles. Minnesota:
Cambridge University Press, 2014. Book.
Tmh. Target 2011: Chemistry 12. Berlin: Tata McGraw-Hill Eduction, 2011. Book.
venkataraman, K. The Chemistry of Synthetic Dyes. London: Elsevier, 2012. Book.
Works Cited
Brian Berkowitz, Ishal Dror, Bruno Yaron. Contaminant Geochemistry. Los Angeles: Springer
Science & Business, 2014. Book.
Cooper, Alan. Biophysical Chemistry. Manchester: Royal Society of Chemistry, 2011. Book.
John Leonard, Barry Lygo, Garry Procter. Advanced Practical Organic Chemistry. New York:
CRC Press, 2013. Book.
Jonathan Clayden, Nick Greeves, Stuart Warren. Organic Chemistry. New York: OUP Oxford,
2012. Book.
Laboratory Manual
Sirkar, Kamalesh. Separation of Molecules, Macromolecules and Particles. Minnesota:
Cambridge University Press, 2014. Book.
Tmh. Target 2011: Chemistry 12. Berlin: Tata McGraw-Hill Eduction, 2011. Book.
venkataraman, K. The Chemistry of Synthetic Dyes. London: Elsevier, 2012. Book.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.