Comprehensive Analysis of Organic Chemistry Reactions and Mechanisms
VerifiedAdded on 2022/08/21
|5
|1159
|8
Homework Assignment
AI Summary
This assignment solution delves into various aspects of organic chemistry, including the reactivity of aromatic compounds and their electrophilic substitution reactions, contrasting them with the behavior of aryl halides in nucleophilic reactions. It explores the sulfonation of naphthalene, detailing the influence of temperature on the formation of different isomers. The solution also examines the mechanisms of E1 and E2 reactions, highlighting the factors that govern their rates and stereochemistry. Furthermore, it discusses Hammond's postulate in the context of radical reactions like chlorination and bromination, and provides insights into primary and secondary kinetic isotope effects, including the nitrogen isotope effect. Finally, the solution addresses reductive alkylation and enantioselective reduction reactions, including the Clarke-Eechweiler method and the reduction of methyl 2,2-dimethyl-3-oxobutanoate, respectively, offering a comprehensive overview of key concepts in organic chemistry.
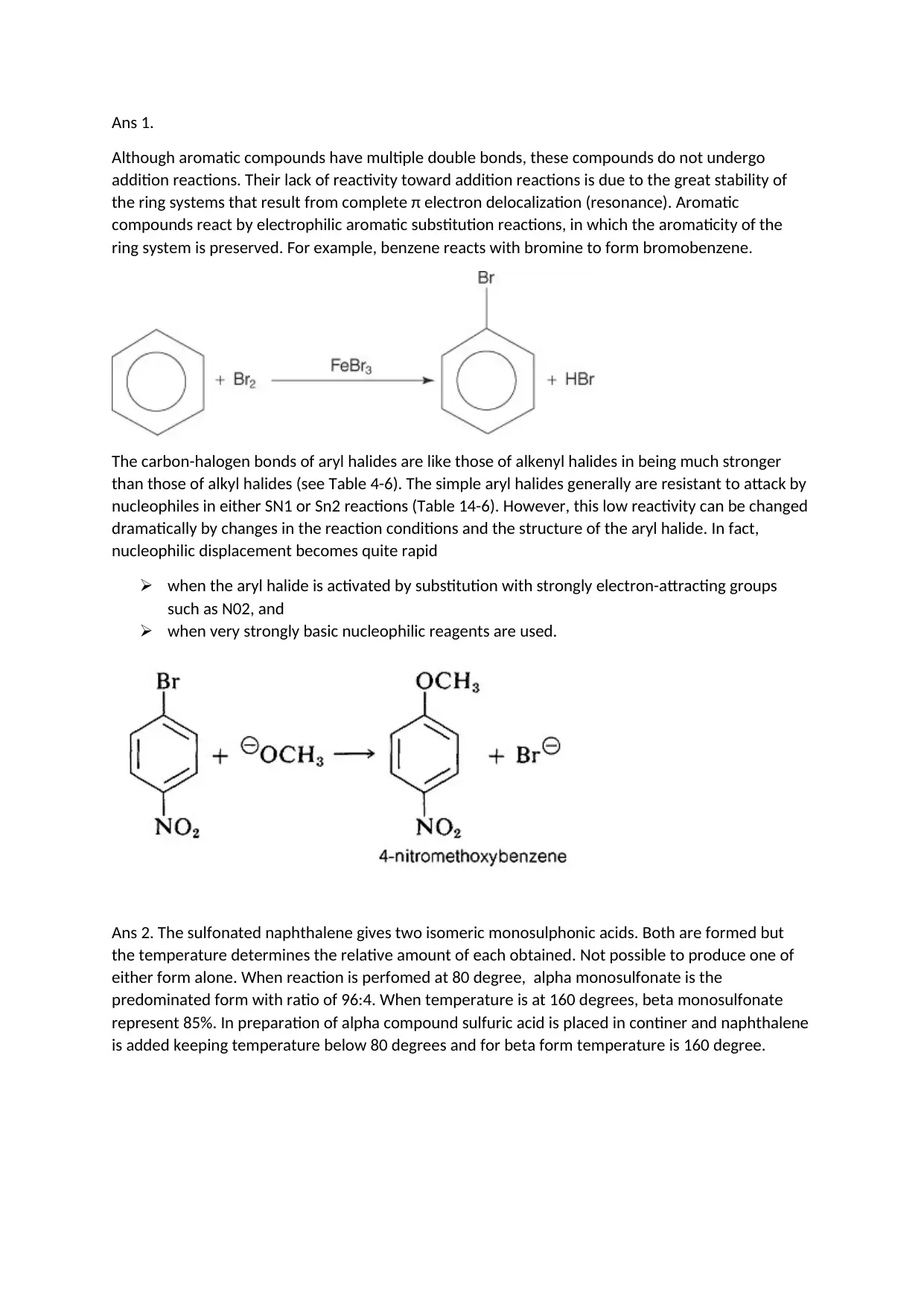
Ans 1.
Although aromatic compounds have multiple double bonds, these compounds do not undergo
addition reactions. Their lack of reactivity toward addition reactions is due to the great stability of
the ring systems that result from complete π electron delocalization (resonance). Aromatic
compounds react by electrophilic aromatic substitution reactions, in which the aromaticity of the
ring system is preserved. For example, benzene reacts with bromine to form bromobenzene.
The carbon-halogen bonds of aryl halides are like those of alkenyl halides in being much stronger
than those of alkyl halides (see Table 4-6). The simple aryl halides generally are resistant to attack by
nucleophiles in either SN1 or Sn2 reactions (Table 14-6). However, this low reactivity can be changed
dramatically by changes in the reaction conditions and the structure of the aryl halide. In fact,
nucleophilic displacement becomes quite rapid
when the aryl halide is activated by substitution with strongly electron-attracting groups
such as N02, and
when very strongly basic nucleophilic reagents are used.
Ans 2. The sulfonated naphthalene gives two isomeric monosulphonic acids. Both are formed but
the temperature determines the relative amount of each obtained. Not possible to produce one of
either form alone. When reaction is perfomed at 80 degree, alpha monosulfonate is the
predominated form with ratio of 96:4. When temperature is at 160 degrees, beta monosulfonate
represent 85%. In preparation of alpha compound sulfuric acid is placed in continer and naphthalene
is added keeping temperature below 80 degrees and for beta form temperature is 160 degree.
Although aromatic compounds have multiple double bonds, these compounds do not undergo
addition reactions. Their lack of reactivity toward addition reactions is due to the great stability of
the ring systems that result from complete π electron delocalization (resonance). Aromatic
compounds react by electrophilic aromatic substitution reactions, in which the aromaticity of the
ring system is preserved. For example, benzene reacts with bromine to form bromobenzene.
The carbon-halogen bonds of aryl halides are like those of alkenyl halides in being much stronger
than those of alkyl halides (see Table 4-6). The simple aryl halides generally are resistant to attack by
nucleophiles in either SN1 or Sn2 reactions (Table 14-6). However, this low reactivity can be changed
dramatically by changes in the reaction conditions and the structure of the aryl halide. In fact,
nucleophilic displacement becomes quite rapid
when the aryl halide is activated by substitution with strongly electron-attracting groups
such as N02, and
when very strongly basic nucleophilic reagents are used.
Ans 2. The sulfonated naphthalene gives two isomeric monosulphonic acids. Both are formed but
the temperature determines the relative amount of each obtained. Not possible to produce one of
either form alone. When reaction is perfomed at 80 degree, alpha monosulfonate is the
predominated form with ratio of 96:4. When temperature is at 160 degrees, beta monosulfonate
represent 85%. In preparation of alpha compound sulfuric acid is placed in continer and naphthalene
is added keeping temperature below 80 degrees and for beta form temperature is 160 degree.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
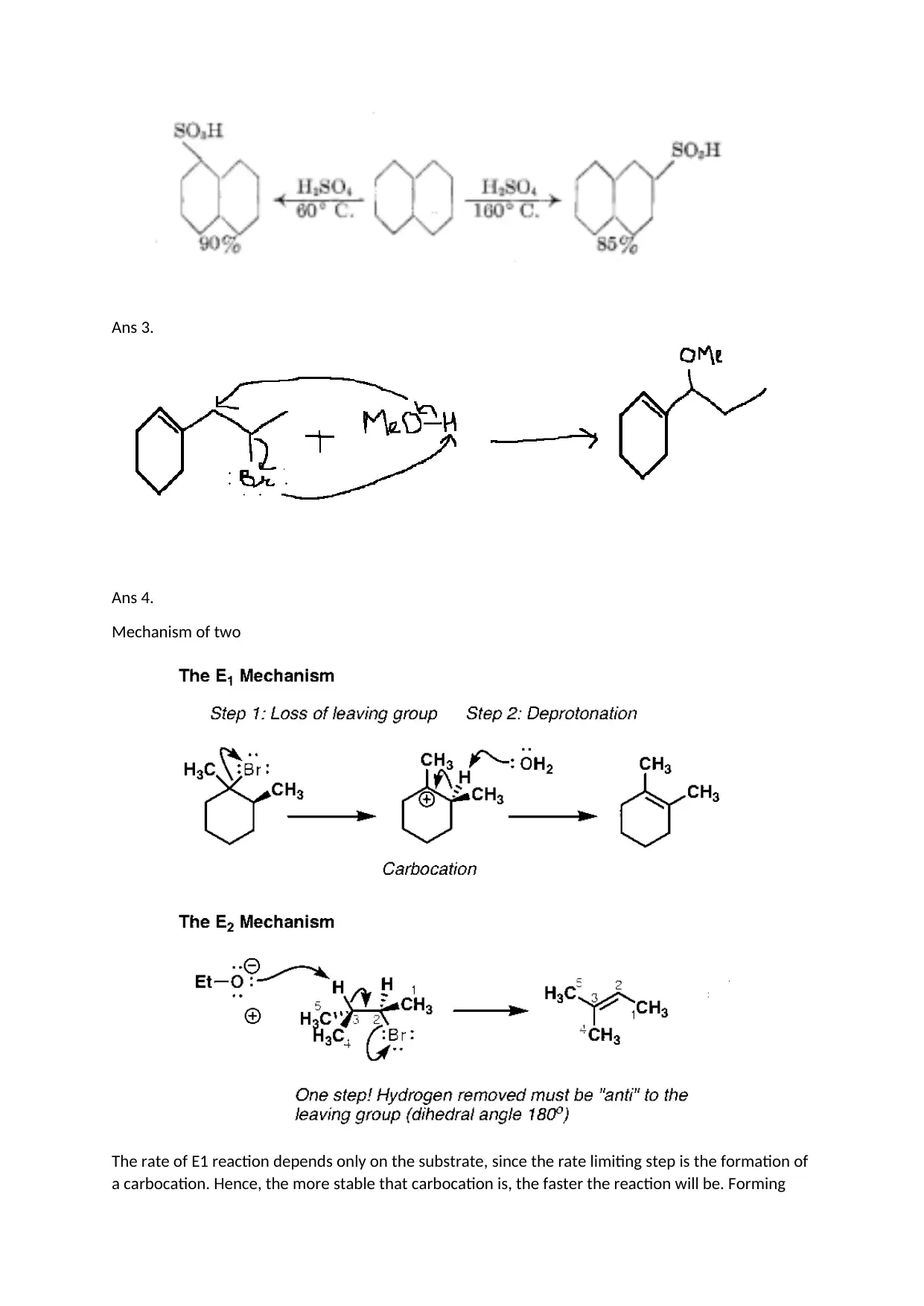
Ans 3.
Ans 4.
Mechanism of two
The rate of E1 reaction depends only on the substrate, since the rate limiting step is the formation of
a carbocation. Hence, the more stable that carbocation is, the faster the reaction will be. Forming
Ans 4.
Mechanism of two
The rate of E1 reaction depends only on the substrate, since the rate limiting step is the formation of
a carbocation. Hence, the more stable that carbocation is, the faster the reaction will be. Forming
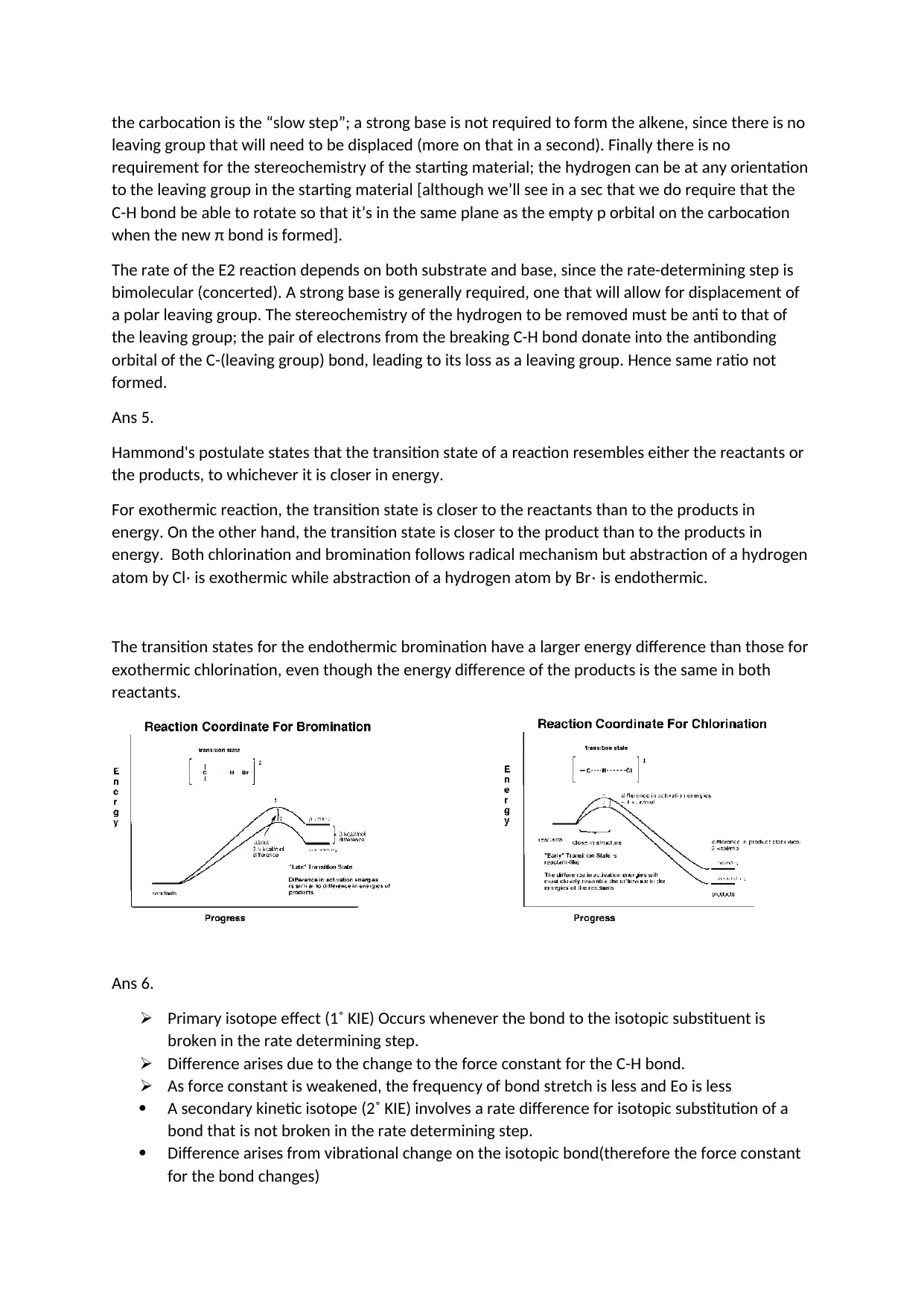
the carbocation is the “slow step”; a strong base is not required to form the alkene, since there is no
leaving group that will need to be displaced (more on that in a second). Finally there is no
requirement for the stereochemistry of the starting material; the hydrogen can be at any orientation
to the leaving group in the starting material [although we’ll see in a sec that we do require that the
C-H bond be able to rotate so that it’s in the same plane as the empty p orbital on the carbocation
when the new π bond is formed].
The rate of the E2 reaction depends on both substrate and base, since the rate-determining step is
bimolecular (concerted). A strong base is generally required, one that will allow for displacement of
a polar leaving group. The stereochemistry of the hydrogen to be removed must be anti to that of
the leaving group; the pair of electrons from the breaking C-H bond donate into the antibonding
orbital of the C-(leaving group) bond, leading to its loss as a leaving group. Hence same ratio not
formed.
Ans 5.
Hammond's postulate states that the transition state of a reaction resembles either the reactants or
the products, to whichever it is closer in energy.
For exothermic reaction, the transition state is closer to the reactants than to the products in
energy. On the other hand, the transition state is closer to the product than to the products in
energy. Both chlorination and bromination follows radical mechanism but abstraction of a hydrogen
atom by Cl⋅ is exothermic while abstraction of a hydrogen atom by Br⋅ is endothermic.
The transition states for the endothermic bromination have a larger energy difference than those for
exothermic chlorination, even though the energy difference of the products is the same in both
reactants.
Ans 6.
Primary isotope effect (1˚ KIE) Occurs whenever the bond to the isotopic substituent is
broken in the rate determining step.
Difference arises due to the change to the force constant for the C-H bond.
As force constant is weakened, the frequency of bond stretch is less and Eo is less
A secondary kinetic isotope (2˚ KIE) involves a rate difference for isotopic substitution of a
bond that is not broken in the rate determining step.
Difference arises from vibrational change on the isotopic bond(therefore the force constant
for the bond changes)
leaving group that will need to be displaced (more on that in a second). Finally there is no
requirement for the stereochemistry of the starting material; the hydrogen can be at any orientation
to the leaving group in the starting material [although we’ll see in a sec that we do require that the
C-H bond be able to rotate so that it’s in the same plane as the empty p orbital on the carbocation
when the new π bond is formed].
The rate of the E2 reaction depends on both substrate and base, since the rate-determining step is
bimolecular (concerted). A strong base is generally required, one that will allow for displacement of
a polar leaving group. The stereochemistry of the hydrogen to be removed must be anti to that of
the leaving group; the pair of electrons from the breaking C-H bond donate into the antibonding
orbital of the C-(leaving group) bond, leading to its loss as a leaving group. Hence same ratio not
formed.
Ans 5.
Hammond's postulate states that the transition state of a reaction resembles either the reactants or
the products, to whichever it is closer in energy.
For exothermic reaction, the transition state is closer to the reactants than to the products in
energy. On the other hand, the transition state is closer to the product than to the products in
energy. Both chlorination and bromination follows radical mechanism but abstraction of a hydrogen
atom by Cl⋅ is exothermic while abstraction of a hydrogen atom by Br⋅ is endothermic.
The transition states for the endothermic bromination have a larger energy difference than those for
exothermic chlorination, even though the energy difference of the products is the same in both
reactants.
Ans 6.
Primary isotope effect (1˚ KIE) Occurs whenever the bond to the isotopic substituent is
broken in the rate determining step.
Difference arises due to the change to the force constant for the C-H bond.
As force constant is weakened, the frequency of bond stretch is less and Eo is less
A secondary kinetic isotope (2˚ KIE) involves a rate difference for isotopic substitution of a
bond that is not broken in the rate determining step.
Difference arises from vibrational change on the isotopic bond(therefore the force constant
for the bond changes)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Changing the force constant affects the shape of the vibrational coordinate
Ans 7.
The results of the nitrogen isotope effect study on the 2-arylethyl system, Table 2, show a
relationship between the magnitude of the effect and the electron-withdrawing or -donating ability
of the para substituent. Although the effects for the p-H and p-OCH, compounds are the same within
experimental error there is a significant decrease in the magnitude of the effect when electron-
withdrawing substituents are placed on the benzene ring.
Ans 8.
The magnitude of the nitrogen effect depends on the extent to which the composite + force
constant of the C-N bond is decreased in going from the initial to the transition state, the effect will
increase regularly in a reaction series with the extent of bond weakening at the transition state.
Consequently, the experimental results show that the extent of carbon-nitrogen bond rupture in the
transition state decreases as the para substituent becomes more electron withdrawing.
Ans 9.
The introduction of methyl groups into a primary or tert. tert-Ba secondary amine by reductive
alkylation with form- aldehyde and formic acid derivatives (the Clarke- 0 Eechweiler method*) has
proved to be a useful method for the preparation of tertiary methylated amines. In tert-Bu —tert-Bu
some cases, however, complex mixtures have resulted 5 from the multiplicity of side reactions which
can occur.’ Our need for a milder procedure in connection with By oxidation of 3b with 6-manganesc
dioxide in40% another problem currently under investigation, cou- aqueous sulfuric acid containing
methanol (1.5/1 w/w) pled with our earlier interest in the chemistry of the at 55° for 4 hr, 2,6-di-
tert-buty]-4-methoxy-4-methyl- _ cyanoborohydride (BHs:CN~-)¢ ion, led us to examine 2,5-
cyelohexadien-l-one (4b) was obtained in 60% the feasibility of a formaldehyde-cyanoborohydride
yield.
Ans 10.
Ans 7.
The results of the nitrogen isotope effect study on the 2-arylethyl system, Table 2, show a
relationship between the magnitude of the effect and the electron-withdrawing or -donating ability
of the para substituent. Although the effects for the p-H and p-OCH, compounds are the same within
experimental error there is a significant decrease in the magnitude of the effect when electron-
withdrawing substituents are placed on the benzene ring.
Ans 8.
The magnitude of the nitrogen effect depends on the extent to which the composite + force
constant of the C-N bond is decreased in going from the initial to the transition state, the effect will
increase regularly in a reaction series with the extent of bond weakening at the transition state.
Consequently, the experimental results show that the extent of carbon-nitrogen bond rupture in the
transition state decreases as the para substituent becomes more electron withdrawing.
Ans 9.
The introduction of methyl groups into a primary or tert. tert-Ba secondary amine by reductive
alkylation with form- aldehyde and formic acid derivatives (the Clarke- 0 Eechweiler method*) has
proved to be a useful method for the preparation of tertiary methylated amines. In tert-Bu —tert-Bu
some cases, however, complex mixtures have resulted 5 from the multiplicity of side reactions which
can occur.’ Our need for a milder procedure in connection with By oxidation of 3b with 6-manganesc
dioxide in40% another problem currently under investigation, cou- aqueous sulfuric acid containing
methanol (1.5/1 w/w) pled with our earlier interest in the chemistry of the at 55° for 4 hr, 2,6-di-
tert-buty]-4-methoxy-4-methyl- _ cyanoborohydride (BHs:CN~-)¢ ion, led us to examine 2,5-
cyelohexadien-l-one (4b) was obtained in 60% the feasibility of a formaldehyde-cyanoborohydride
yield.
Ans 10.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
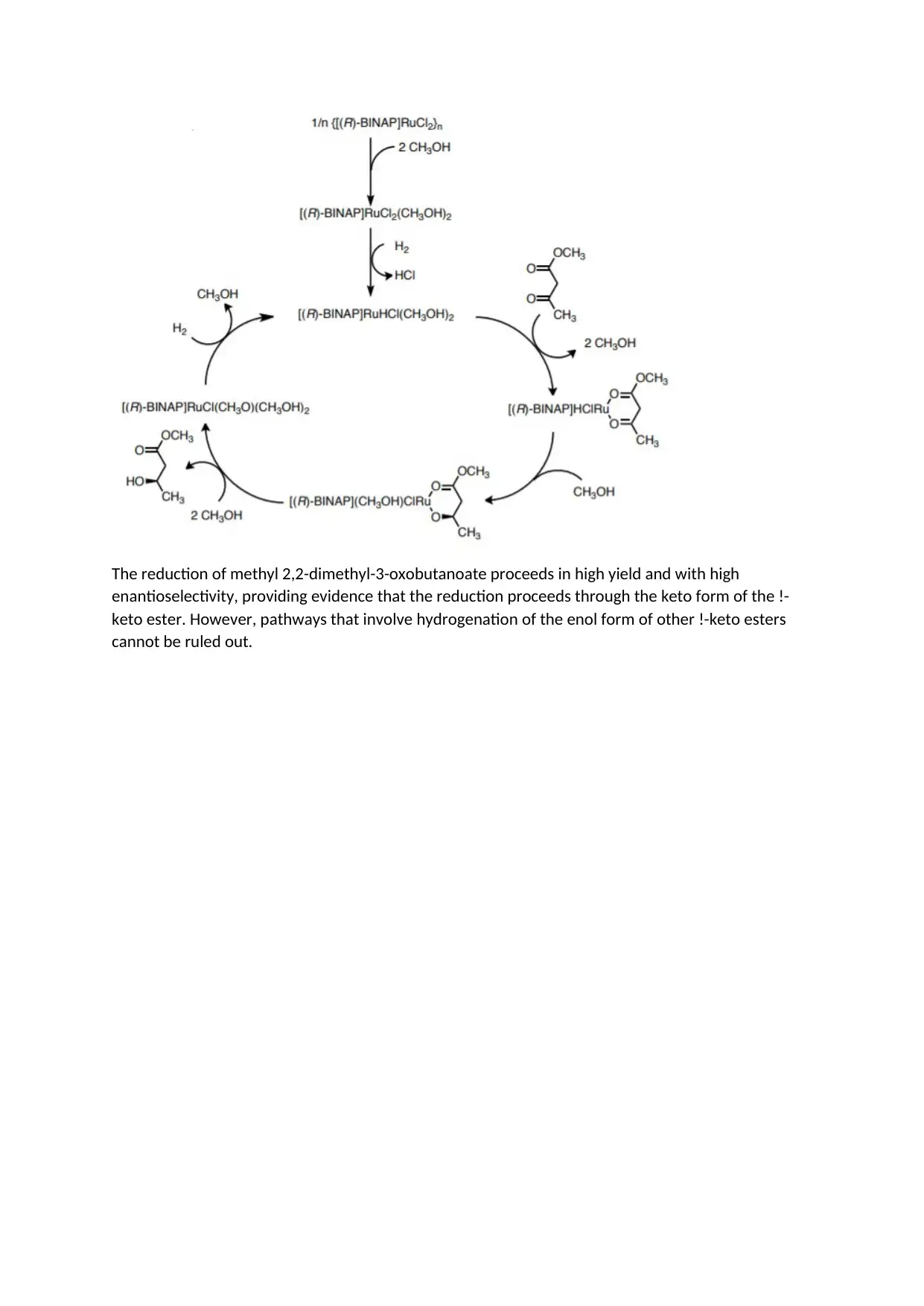
The reduction of methyl 2,2-dimethyl-3-oxobutanoate proceeds in high yield and with high
enantioselectivity, providing evidence that the reduction proceeds through the keto form of the !-
keto ester. However, pathways that involve hydrogenation of the enol form of other !-keto esters
cannot be ruled out.
enantioselectivity, providing evidence that the reduction proceeds through the keto form of the !-
keto ester. However, pathways that involve hydrogenation of the enol form of other !-keto esters
cannot be ruled out.
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.