Organic Chemistry Lab Reports: Experiments and Analysis
VerifiedAdded on 2023/06/04
|37
|5489
|304
Report
AI Summary
This document presents three organic chemistry lab reports. The first report investigates the chemical properties of aldehydes and ketones, focusing on differentiation tests and the formation of derivatives, with detailed background information, experimental procedures, and pre- and post-lab questions. The second report details the synthesis of Trans-9-(2-phenylethenyl)-anthracene via the Wittig reaction, including background information, reaction mechanisms, and pre-lab questions. The third report covers esterification, outlining the experimental procedure using a -COOH acid and alcohols with an acid catalyst. Each report includes reactants, products, properties, and relevant figures, providing a comprehensive overview of the experiments and their outcomes. The reports cover topics like identifying ketones and aldehydes and using Wittig reactions for alkene synthesis.

Running head: ORGANIC CHEMISTRY LAB REPORTS
1
Organic chemistry lab reports
Name:
Institution:
1
Organic chemistry lab reports
Name:
Institution:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ORGANIC CHEMISTRY LAB REPORTS 2
First Lab report
Purpose of the experiment
The aim of the experiment is to investigate the chemical properties of aldehydes and ketone
representatives. The experiment uses these chemical physiognomies in a simple test to
differentiate between ketones and aldehydes. Also, experiment aim to identify ketones and
aldehydes by the formation of derivatives.
Background information
Aldehydes are group of organic mixtures in which a carbon fragment shares a double
bond with oxygen. The double bond between oxygen and carbon is characteristic of all the
aldehydes. Aldehydes can undergo a number of reactions. A key similarity in the reactions of
aldehydes is that carbonyl is the site of the reactivity. The carbonyl carbon of the compounds is
slightly positive due to its double bonding to electronegative oxygen. Therefore, the double bond
makes the carbonyl carbon electrophilic. When the compound reacts with a nucleophile, the
nucleophile creates a new bond with the carbonyl carbon. Molecules that act as a nucleophile
typically have lone of electron ready to attack the carbonyl carbon.
Ketones and aldehydes are illustrative of complexes that have the carbonyl groups.
Aldehydes have at least one of hydrogen linked to the carbonyl carbon while in ketones no
hydrogen’s is joined to the carbonyl carbon. Aldehydes and ketone with low molecular weight
have commercial benefits.
Aldehydes are easily oxidized to a carboxylic acid. This hydrogen is activated by a
carbonyl group and is readily oxidized to OH group. Ketones have no hydrogen joined directly to
First Lab report
Purpose of the experiment
The aim of the experiment is to investigate the chemical properties of aldehydes and ketone
representatives. The experiment uses these chemical physiognomies in a simple test to
differentiate between ketones and aldehydes. Also, experiment aim to identify ketones and
aldehydes by the formation of derivatives.
Background information
Aldehydes are group of organic mixtures in which a carbon fragment shares a double
bond with oxygen. The double bond between oxygen and carbon is characteristic of all the
aldehydes. Aldehydes can undergo a number of reactions. A key similarity in the reactions of
aldehydes is that carbonyl is the site of the reactivity. The carbonyl carbon of the compounds is
slightly positive due to its double bonding to electronegative oxygen. Therefore, the double bond
makes the carbonyl carbon electrophilic. When the compound reacts with a nucleophile, the
nucleophile creates a new bond with the carbonyl carbon. Molecules that act as a nucleophile
typically have lone of electron ready to attack the carbonyl carbon.
Ketones and aldehydes are illustrative of complexes that have the carbonyl groups.
Aldehydes have at least one of hydrogen linked to the carbonyl carbon while in ketones no
hydrogen’s is joined to the carbonyl carbon. Aldehydes and ketone with low molecular weight
have commercial benefits.
Aldehydes are easily oxidized to a carboxylic acid. This hydrogen is activated by a
carbonyl group and is readily oxidized to OH group. Ketones have no hydrogen joined directly to

ORGANIC CHEMISTRY LAB REPORTS 3
a carbonyl group and do not readily oxidize and have no effect on mild oxidizing agent. Strong
oxidizers like hot concentrated nitric acid can oxidize ketone breaking, a -C-C- bond
forming at least two molecules of carboxylic
Aldehydes are oxidized by the chromic acid to carboxylic acid whereas ketones are not.
The positive outcomes involve the creation of the blue-green color solution from the brown-red
of chromic acid (Bettelheim & Landesberg, 2012, pp. 295). The Tollen's reagent is a solution of
complex silver ion in alkaline solution. When aldehyde are warmed with Tollen reagent, a
metallic silver precipitate is produced and left on the walls of test tubes giving a silver mirror.
Most aldehydes reduce Tollen’s reagent to provide a precipitous of silver metal. The
unrestricted silver creates a silver mirror on the flanks of the beaker (Bettelheim & Landesberg,
2012, pp. 296).
Figure 1: (Bettelheim & Landesberg, 2012, pp. 296).
Methyl ketones provide the yellow precipitous iodoform when they react with I2 in
aqueous NaOH. Aldehydes and ketones give an instant precipitous with 2, 4-
dinitrophenylhydrazine reagents. The color of precipitous differs from yellow to red.
a carbonyl group and do not readily oxidize and have no effect on mild oxidizing agent. Strong
oxidizers like hot concentrated nitric acid can oxidize ketone breaking, a -C-C- bond
forming at least two molecules of carboxylic
Aldehydes are oxidized by the chromic acid to carboxylic acid whereas ketones are not.
The positive outcomes involve the creation of the blue-green color solution from the brown-red
of chromic acid (Bettelheim & Landesberg, 2012, pp. 295). The Tollen's reagent is a solution of
complex silver ion in alkaline solution. When aldehyde are warmed with Tollen reagent, a
metallic silver precipitate is produced and left on the walls of test tubes giving a silver mirror.
Most aldehydes reduce Tollen’s reagent to provide a precipitous of silver metal. The
unrestricted silver creates a silver mirror on the flanks of the beaker (Bettelheim & Landesberg,
2012, pp. 296).
Figure 1: (Bettelheim & Landesberg, 2012, pp. 296).
Methyl ketones provide the yellow precipitous iodoform when they react with I2 in
aqueous NaOH. Aldehydes and ketones give an instant precipitous with 2, 4-
dinitrophenylhydrazine reagents. The color of precipitous differs from yellow to red.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ORGANIC CHEMISTRY LAB REPORTS 4
Figure 2: (Bettelheim & Landesberg, 2012, pp. 297)
The classification test, when properly done, can differentiate between numerous sorts of
ketones and aldehydes. These experiments only may not permit for the identification of
particular unidentified ketones or ketones. A method to appropriately classify an unfamiliar
compound is by using a well-known chemical reaction to change it into another complex that is
identified. The ideal derivative is solid. A solid can be certainly refined by the crystallization and
easily characterized by its melting point. Thus, two identical aldehydes or ketones typically have
a derivative that has various melting points. The most often generated derivatives for ketones and
aldehydes are 2, 4-dintrophenylhydraone, oxime, and Semicarbazone (Bettelheim & Landesberg,
2012, pp. 297).
Figure 2: (Bettelheim & Landesberg, 2012, pp. 297)
The classification test, when properly done, can differentiate between numerous sorts of
ketones and aldehydes. These experiments only may not permit for the identification of
particular unidentified ketones or ketones. A method to appropriately classify an unfamiliar
compound is by using a well-known chemical reaction to change it into another complex that is
identified. The ideal derivative is solid. A solid can be certainly refined by the crystallization and
easily characterized by its melting point. Thus, two identical aldehydes or ketones typically have
a derivative that has various melting points. The most often generated derivatives for ketones and
aldehydes are 2, 4-dintrophenylhydraone, oxime, and Semicarbazone (Bettelheim & Landesberg,
2012, pp. 297).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
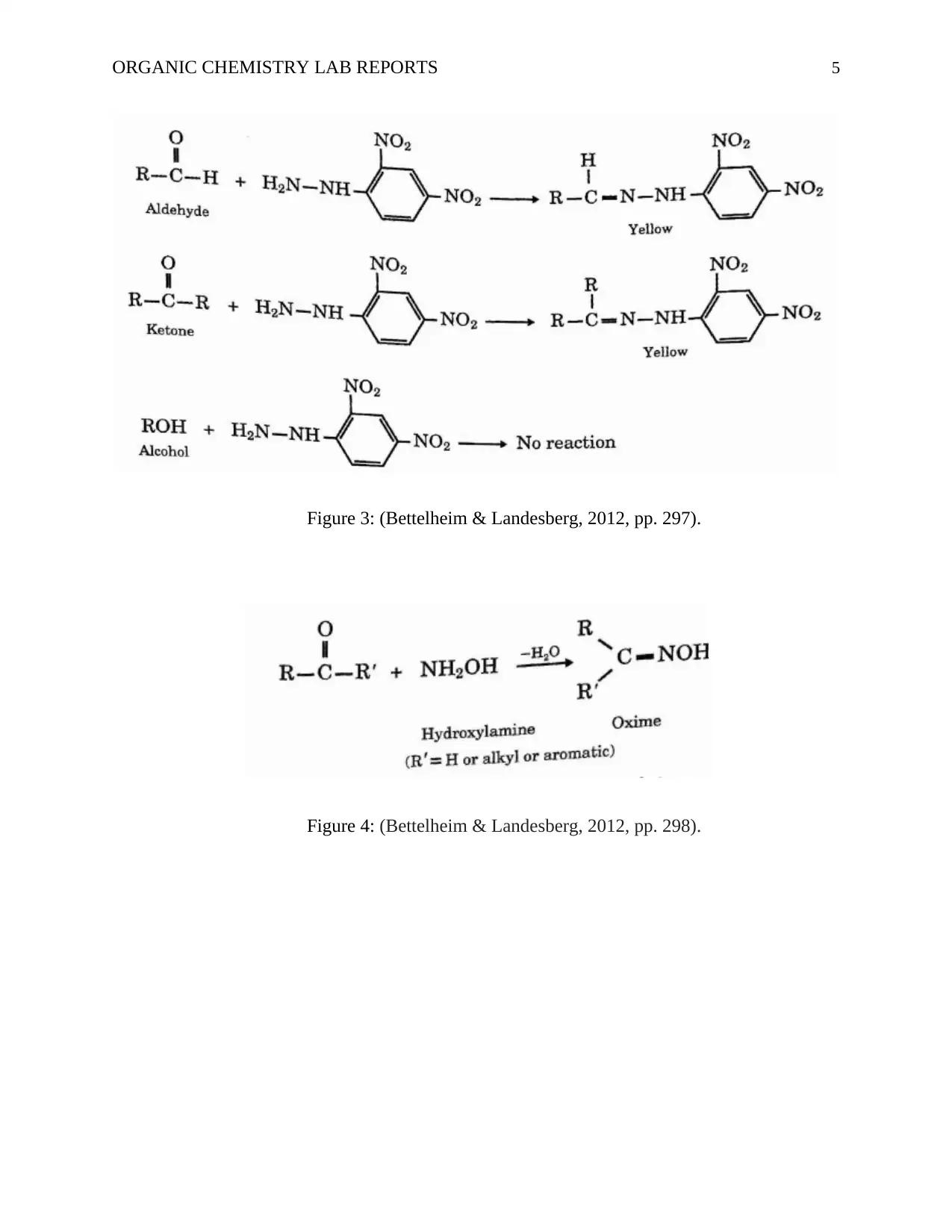
ORGANIC CHEMISTRY LAB REPORTS 5
Figure 3: (Bettelheim & Landesberg, 2012, pp. 297).
Figure 4: (Bettelheim & Landesberg, 2012, pp. 298).
Figure 3: (Bettelheim & Landesberg, 2012, pp. 297).
Figure 4: (Bettelheim & Landesberg, 2012, pp. 298).
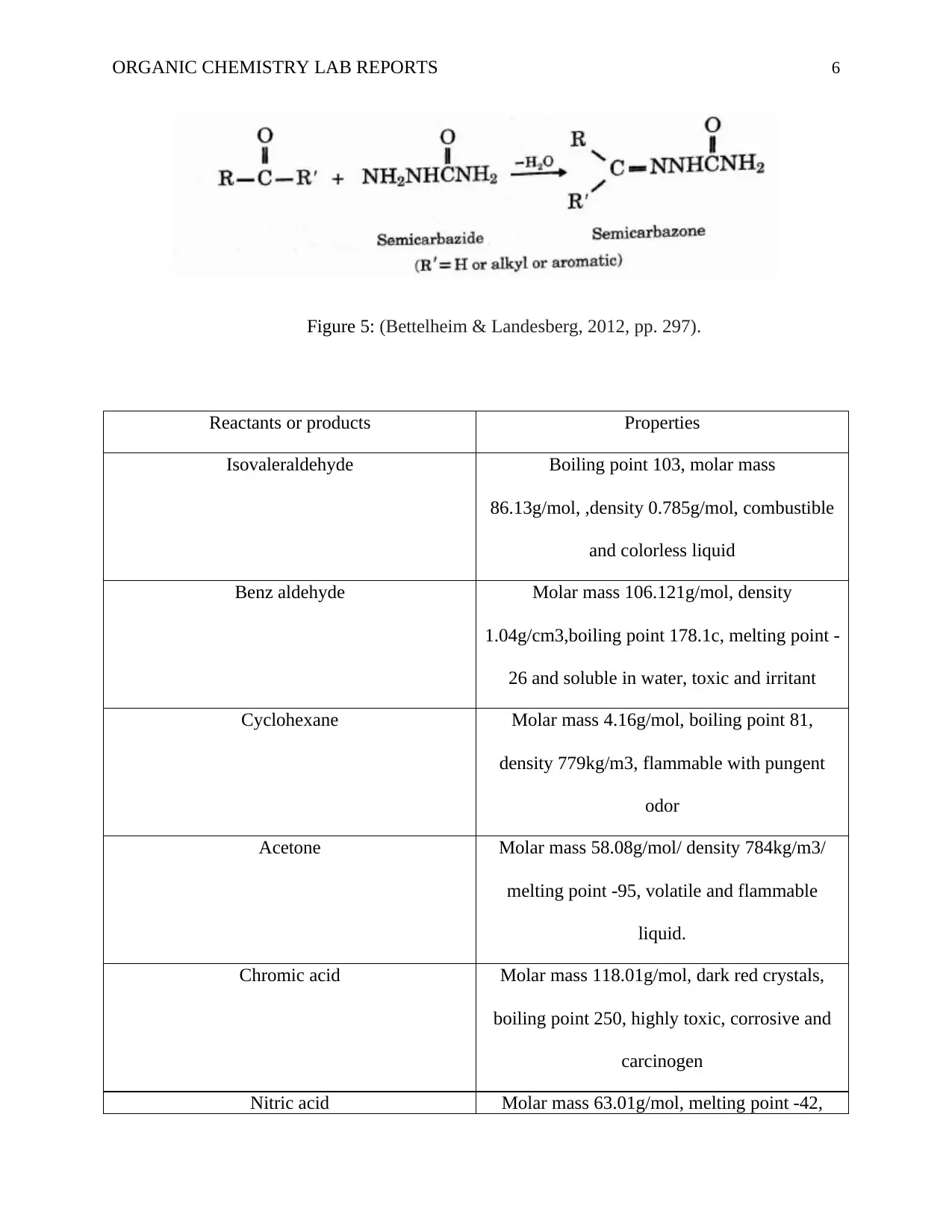
ORGANIC CHEMISTRY LAB REPORTS 6
Figure 5: (Bettelheim & Landesberg, 2012, pp. 297).
Reactants or products Properties
Isovaleraldehyde Boiling point 103, molar mass
86.13g/mol, ,density 0.785g/mol, combustible
and colorless liquid
Benz aldehyde Molar mass 106.121g/mol, density
1.04g/cm3,boiling point 178.1c, melting point -
26 and soluble in water, toxic and irritant
Cyclohexane Molar mass 4.16g/mol, boiling point 81,
density 779kg/m3, flammable with pungent
odor
Acetone Molar mass 58.08g/mol/ density 784kg/m3/
melting point -95, volatile and flammable
liquid.
Chromic acid Molar mass 118.01g/mol, dark red crystals,
boiling point 250, highly toxic, corrosive and
carcinogen
Nitric acid Molar mass 63.01g/mol, melting point -42,
Figure 5: (Bettelheim & Landesberg, 2012, pp. 297).
Reactants or products Properties
Isovaleraldehyde Boiling point 103, molar mass
86.13g/mol, ,density 0.785g/mol, combustible
and colorless liquid
Benz aldehyde Molar mass 106.121g/mol, density
1.04g/cm3,boiling point 178.1c, melting point -
26 and soluble in water, toxic and irritant
Cyclohexane Molar mass 4.16g/mol, boiling point 81,
density 779kg/m3, flammable with pungent
odor
Acetone Molar mass 58.08g/mol/ density 784kg/m3/
melting point -95, volatile and flammable
liquid.
Chromic acid Molar mass 118.01g/mol, dark red crystals,
boiling point 250, highly toxic, corrosive and
carcinogen
Nitric acid Molar mass 63.01g/mol, melting point -42,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
ORGANIC CHEMISTRY LAB REPORTS 7
boiling point 83, density 1.51g/cm3, highly
corrosive mineral acid.
2-4 dinitrophenyl hydrazine Orange or red powder, molar mass
198.14g/mol, melting point 198-202, slightly
soluble in water, flammable and possibly
carcinogenic
Sodium hydroxide Molar mass 39.997g/mol, density 2.13g/cm3,
soluble in ethanol, water, and methanol, very
corrosive
1,2-dimethoxyethane Boiling point 85, density 868kg/m3, molar
mass 90.12g/mol, melting point -58, toxic by
inhalation and ingestion
Methanol Molar mass 32.04g/mol, boiling point 64.7,
density 792kg/m3, melting point -97.6,
colorless, flammable, volatile and poisonous
liquid.
Pyridine Molar mass 79.1g/mol, density 982kg/m3,
boiling point 115, toxic
Pre-lab question
boiling point 83, density 1.51g/cm3, highly
corrosive mineral acid.
2-4 dinitrophenyl hydrazine Orange or red powder, molar mass
198.14g/mol, melting point 198-202, slightly
soluble in water, flammable and possibly
carcinogenic
Sodium hydroxide Molar mass 39.997g/mol, density 2.13g/cm3,
soluble in ethanol, water, and methanol, very
corrosive
1,2-dimethoxyethane Boiling point 85, density 868kg/m3, molar
mass 90.12g/mol, melting point -58, toxic by
inhalation and ingestion
Methanol Molar mass 32.04g/mol, boiling point 64.7,
density 792kg/m3, melting point -97.6,
colorless, flammable, volatile and poisonous
liquid.
Pyridine Molar mass 79.1g/mol, density 982kg/m3,
boiling point 115, toxic
Pre-lab question
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
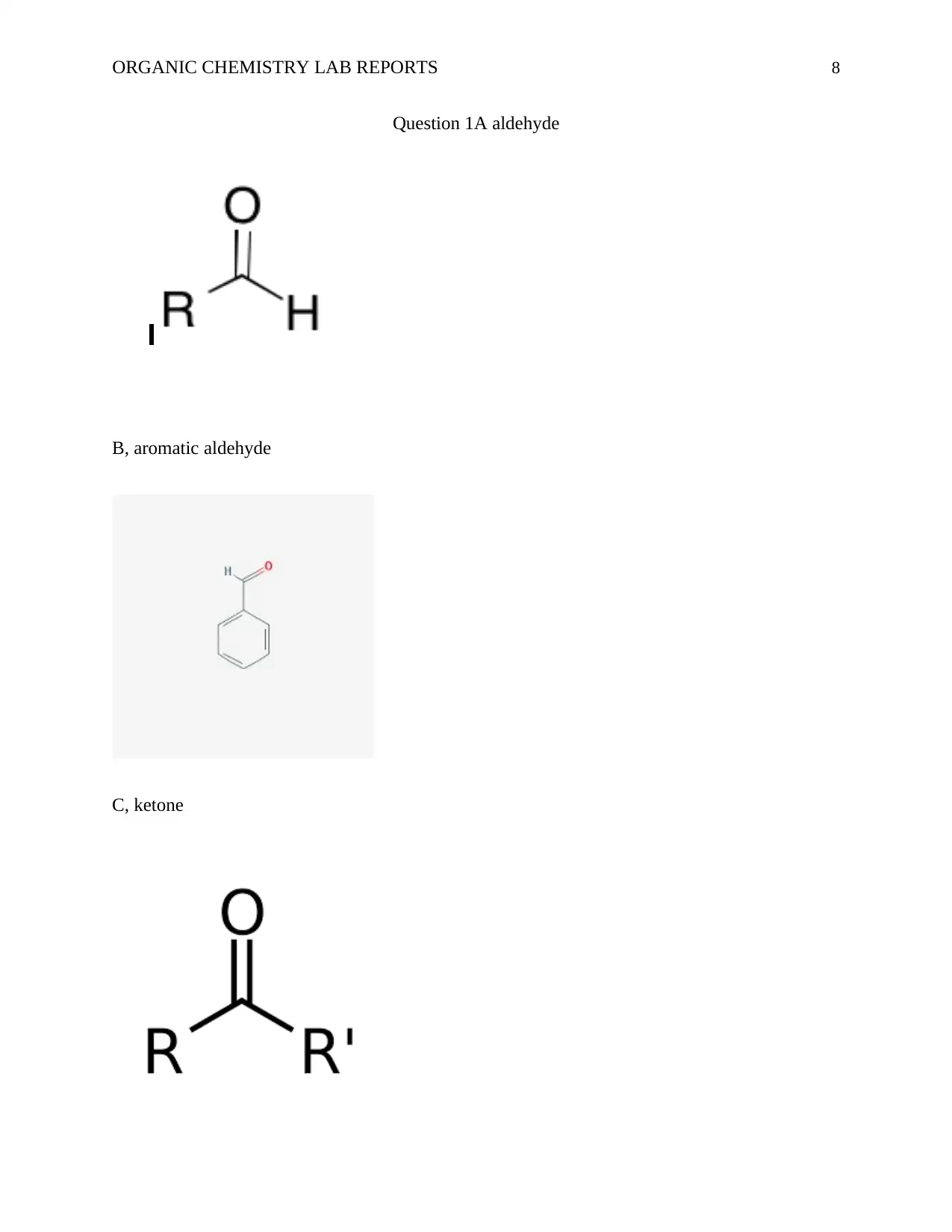
ORGANIC CHEMISTRY LAB REPORTS 8
Question 1A aldehyde
B, aromatic aldehyde
C, ketone
Question 1A aldehyde
B, aromatic aldehyde
C, ketone
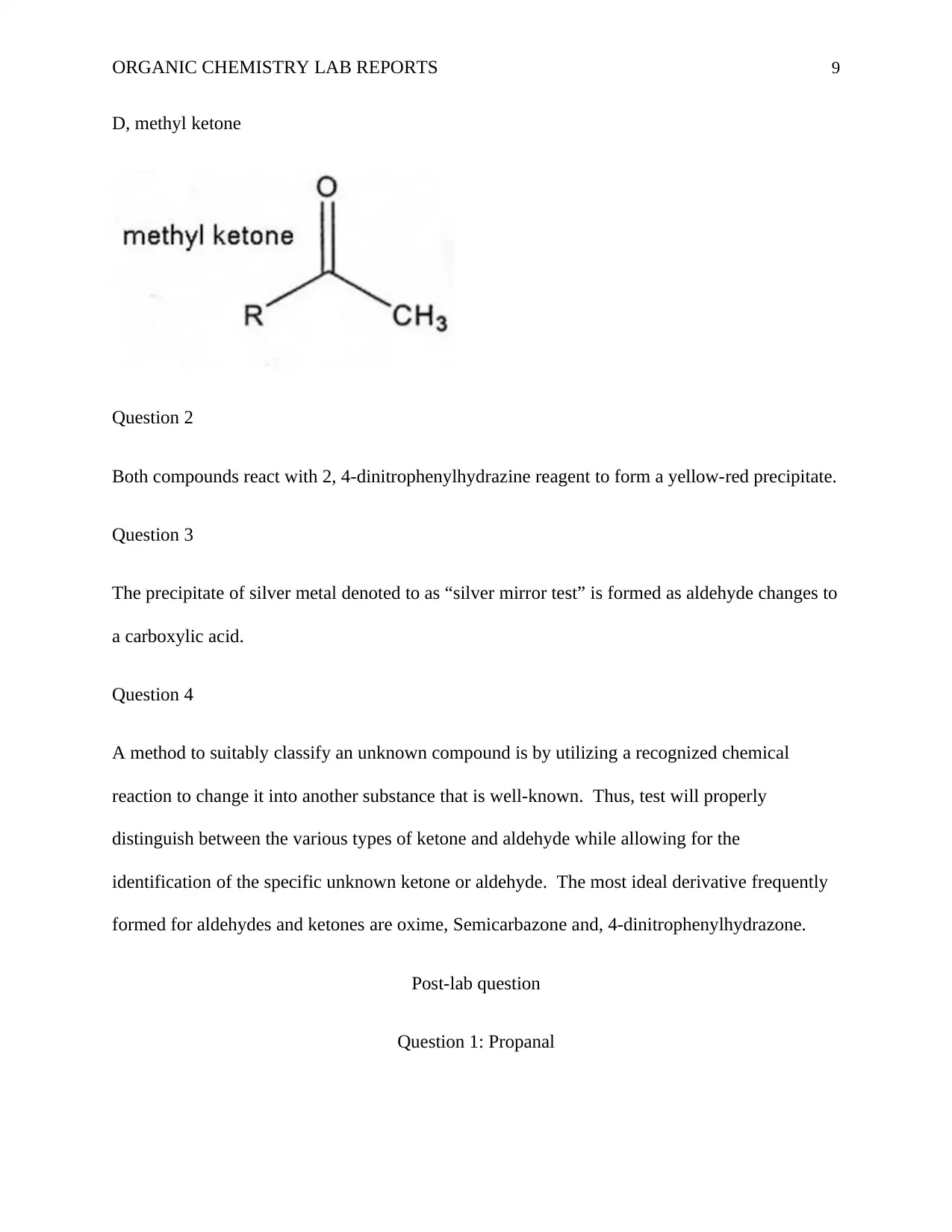
ORGANIC CHEMISTRY LAB REPORTS 9
D, methyl ketone
Question 2
Both compounds react with 2, 4-dinitrophenylhydrazine reagent to form a yellow-red precipitate.
Question 3
The precipitate of silver metal denoted to as “silver mirror test” is formed as aldehyde changes to
a carboxylic acid.
Question 4
A method to suitably classify an unknown compound is by utilizing a recognized chemical
reaction to change it into another substance that is well-known. Thus, test will properly
distinguish between the various types of ketone and aldehyde while allowing for the
identification of the specific unknown ketone or aldehyde. The most ideal derivative frequently
formed for aldehydes and ketones are oxime, Semicarbazone and, 4-dinitrophenylhydrazone.
Post-lab question
Question 1: Propanal
D, methyl ketone
Question 2
Both compounds react with 2, 4-dinitrophenylhydrazine reagent to form a yellow-red precipitate.
Question 3
The precipitate of silver metal denoted to as “silver mirror test” is formed as aldehyde changes to
a carboxylic acid.
Question 4
A method to suitably classify an unknown compound is by utilizing a recognized chemical
reaction to change it into another substance that is well-known. Thus, test will properly
distinguish between the various types of ketone and aldehyde while allowing for the
identification of the specific unknown ketone or aldehyde. The most ideal derivative frequently
formed for aldehydes and ketones are oxime, Semicarbazone and, 4-dinitrophenylhydrazone.
Post-lab question
Question 1: Propanal
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ORGANIC CHEMISTRY LAB REPORTS 10
Question 2a
2-pentanone with 2, 4 dinitrophenylhydrazine forms a yellow-red precipitate
2-pentanone with KI-I2 forms yellow precipitate iodoform
2-pentanone does not reduce Tollen’s reagent. Hence, no reaction occurs
2b
Cyclohexane aldehyde reacts with 2, 4 dinitrophenylhydrazines to form yellow to red precipitate
Cyclohexane aldehyde reacts with chromic acid to form a blue-green from the brown-red color
solution of chromic acid
Cyclohexane aldehyde reacts with Tollen's mixture to create a precipitous of silver metal (silver
mirror test).
Question 3
Even though 2-pentanone and valeraldehyde (pentanal) have a close boiling point,
experimentally, chromic acid can oxidize aldehyde to their respective carboxylic while ketone is
not oxidized by chromic acid. The positive test effects in the creation of a blue-green solution
from the brown-red color solution.
Question 2a
2-pentanone with 2, 4 dinitrophenylhydrazine forms a yellow-red precipitate
2-pentanone with KI-I2 forms yellow precipitate iodoform
2-pentanone does not reduce Tollen’s reagent. Hence, no reaction occurs
2b
Cyclohexane aldehyde reacts with 2, 4 dinitrophenylhydrazines to form yellow to red precipitate
Cyclohexane aldehyde reacts with chromic acid to form a blue-green from the brown-red color
solution of chromic acid
Cyclohexane aldehyde reacts with Tollen's mixture to create a precipitous of silver metal (silver
mirror test).
Question 3
Even though 2-pentanone and valeraldehyde (pentanal) have a close boiling point,
experimentally, chromic acid can oxidize aldehyde to their respective carboxylic while ketone is
not oxidized by chromic acid. The positive test effects in the creation of a blue-green solution
from the brown-red color solution.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ORGANIC CHEMISTRY LAB REPORTS 11
Question 5
Cyclopentane does not show positive tests with chromic acid, Semicarbazide, 2-4
dinitrophenylhydrazine or Tollen reagents. Both Cyclopentanone and cyclo pentanal show
positive test with 2-4 dinitrophenylhydrazine, oxime, and Semicarbazide. However, Tollen
reagent can be used to distinguish the latter two compounds. When cyclo pentanal is warmed
with Tollen reagents, a metallic silver precipitate is formed and deposit on the walls of the
beaker or test tubes giving a silver mirror referred as silver mirror test. But cyclopentanone will
not reduce Tollen reagents
Second lab report
Synthesis of Trans-9-(2-phenylethenyl)-anthracene: Wittig reaction
Aim of the experiment
The aim of the research is to synthesis Trans-9-(2-phenylethenyl) anthracene by a Wittig
reaction. It characterizes the product by nuclear magnetic resonance spectroscopy, thin-layer
chromatography, infrared spectroscopy and melting point. One should be familiar with
techniques for drying organic solvent and measuring melting points. Additionally, one should be
familiar with the techniques for distillation, extraction, recrystallization, and vacuum filtration.
Similarly, one should be acquainted with how to speed the evaporation of micro scale quantities
of solvent using nitrogen or air. Finally, one should be conversant with the nuclear magnetic
resonance spectroscopy, thin-layer chromatography and infrared. In this study, the alkene Trans-
9-(2-phenylethenyl) anthracene will be manufactured from 9-anthraldehyde and the ylide derived
from the triphenylbenzylphosponium chloride.
Question 5
Cyclopentane does not show positive tests with chromic acid, Semicarbazide, 2-4
dinitrophenylhydrazine or Tollen reagents. Both Cyclopentanone and cyclo pentanal show
positive test with 2-4 dinitrophenylhydrazine, oxime, and Semicarbazide. However, Tollen
reagent can be used to distinguish the latter two compounds. When cyclo pentanal is warmed
with Tollen reagents, a metallic silver precipitate is formed and deposit on the walls of the
beaker or test tubes giving a silver mirror referred as silver mirror test. But cyclopentanone will
not reduce Tollen reagents
Second lab report
Synthesis of Trans-9-(2-phenylethenyl)-anthracene: Wittig reaction
Aim of the experiment
The aim of the research is to synthesis Trans-9-(2-phenylethenyl) anthracene by a Wittig
reaction. It characterizes the product by nuclear magnetic resonance spectroscopy, thin-layer
chromatography, infrared spectroscopy and melting point. One should be familiar with
techniques for drying organic solvent and measuring melting points. Additionally, one should be
familiar with the techniques for distillation, extraction, recrystallization, and vacuum filtration.
Similarly, one should be acquainted with how to speed the evaporation of micro scale quantities
of solvent using nitrogen or air. Finally, one should be conversant with the nuclear magnetic
resonance spectroscopy, thin-layer chromatography and infrared. In this study, the alkene Trans-
9-(2-phenylethenyl) anthracene will be manufactured from 9-anthraldehyde and the ylide derived
from the triphenylbenzylphosponium chloride.

ORGANIC CHEMISTRY LAB REPORTS 12
Background information
Chemical reaction comprising organic molecules can be categorized into three huge
classes; elimination, addition and molecular rearrangement. Even though alkenes are made from
elimination such as dehydrogenation of alkyl halide (RX) and alcohols, these reactions normally
effect in a blend of structural isomers. The Wittig reaction is favored as a method of producing
alkenes because of its great point of regioselectivity, where reaction tends to generate one isomer
from a solitary reactant. The reaction assists to choose the precise location of the newly formed
bond. Thus, the reaction is an addition which produces an alkene from the reaction of a
carbonyl complex with a carbon-holding phosphorous substance well-known as ylide, which is
prepared from a phosphonium halide. The phosphonium halide is created from the nucleophilic
substitution of a primary (10) or secondary (20) alkyl halide and triphenylphosphine. Ph3P is a
relatively weak base and good nucleophile. Thus, the probable competing elimination reaction
does not happen
The salt of phosphonium ought to have P linked to a C having at least one H atom. This
H atom is reasonably acidic and can be removed in the strong base. In this experiment, a
concentrated solution of NaOH will be utilized. The resulting product from the reaction of a
strong base and phosphonium halide is referred to as ylide.
The mechanism of carbonyl compound and triphenylphosphonium ylide is still under
study. The argument arises in whether the oxaphosphetane intermediary is created by two or one
phase concentrated procedure. The driving force for the oxaphosphetane decomposition is
thought to be the creation of the strong P-0 bond of the phosphine oxide. The witting process
Background information
Chemical reaction comprising organic molecules can be categorized into three huge
classes; elimination, addition and molecular rearrangement. Even though alkenes are made from
elimination such as dehydrogenation of alkyl halide (RX) and alcohols, these reactions normally
effect in a blend of structural isomers. The Wittig reaction is favored as a method of producing
alkenes because of its great point of regioselectivity, where reaction tends to generate one isomer
from a solitary reactant. The reaction assists to choose the precise location of the newly formed
bond. Thus, the reaction is an addition which produces an alkene from the reaction of a
carbonyl complex with a carbon-holding phosphorous substance well-known as ylide, which is
prepared from a phosphonium halide. The phosphonium halide is created from the nucleophilic
substitution of a primary (10) or secondary (20) alkyl halide and triphenylphosphine. Ph3P is a
relatively weak base and good nucleophile. Thus, the probable competing elimination reaction
does not happen
The salt of phosphonium ought to have P linked to a C having at least one H atom. This
H atom is reasonably acidic and can be removed in the strong base. In this experiment, a
concentrated solution of NaOH will be utilized. The resulting product from the reaction of a
strong base and phosphonium halide is referred to as ylide.
The mechanism of carbonyl compound and triphenylphosphonium ylide is still under
study. The argument arises in whether the oxaphosphetane intermediary is created by two or one
phase concentrated procedure. The driving force for the oxaphosphetane decomposition is
thought to be the creation of the strong P-0 bond of the phosphine oxide. The witting process
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 37
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
