A Comprehensive Report on Organic Alcohols, Aldehydes, Ketones, Acids
VerifiedAdded on 2020/01/28
|7
|1097
|572
Report
AI Summary
This report provides a comprehensive overview of organic alcohols, aldehydes, ketones, and carboxylic acids. It details their structures, including the specific bonding and hybridization of carbon and oxygen atoms within their functional groups (-OH, C=O, and -COOH). The report explains the nomenclature of each compound type using IUPAC rules, providing examples such as ethanol, propanone, and ethanoic acid. It also outlines the physical properties of these compounds, including their states of matter, odors, and boiling points, and discusses the interconversion reactions between these organic compounds, such as the oxidation of alcohols to aldehydes, ketones, or carboxylic acids and the reduction of aldehydes and ketones to alcohols, illustrating how these conversions occur in organic chemistry. The report includes named examples of each compound type and references supporting the provided information.

Chemistry 1
Chemistry
Student's Name:
Instructor's Name:
Date:
Chemistry
Student's Name:
Instructor's Name:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 2
Describe the structure, bonding, physical properties, interconversion and nomenclature of
organic alcohols, aldehydes, ketones and carboxylic acids. Give named examples of each.
There are several types of organic compounds and they vary in their properties, depending upon
the ‘functional group’ that is attached to their structure. Accordingly, the alcohols, aldehydes,
ketones and carbohydrates possess distinct and unique chemical and physical properties due to
these ‘functional groups.’
Introduction:
Compounds, with a hydroxyl (-OH) group attached to a saturated hydrocarbons are called
alcohols (Aaaron, nd).
An alcohol is represented as (Clark, 2003.):
This is ‘Methyl Alcohol’ or Methanol, the simplest of alcohols, with the central carbon atom
surrounded by three hydrogen and one hydroxyl group.
Alcohols can contain one or more hydroxyl group, and are thereby named depending upon the
number of hydroxyl group they possess, as mono, di, tri or polyhydric alcohols.
Aldehydes and ketones both contain C=O group, the carbonyl group. While ketones have the
carbonyl group joined on both ends with one ‘alkyl’ group, the aldehydes have one end attached
to alkyl group, and the other, with a hydrogen.
Example:
Hence, functional group of aldehydes is –CHO, while that of Ketones is C=O.
Describe the structure, bonding, physical properties, interconversion and nomenclature of
organic alcohols, aldehydes, ketones and carboxylic acids. Give named examples of each.
There are several types of organic compounds and they vary in their properties, depending upon
the ‘functional group’ that is attached to their structure. Accordingly, the alcohols, aldehydes,
ketones and carbohydrates possess distinct and unique chemical and physical properties due to
these ‘functional groups.’
Introduction:
Compounds, with a hydroxyl (-OH) group attached to a saturated hydrocarbons are called
alcohols (Aaaron, nd).
An alcohol is represented as (Clark, 2003.):
This is ‘Methyl Alcohol’ or Methanol, the simplest of alcohols, with the central carbon atom
surrounded by three hydrogen and one hydroxyl group.
Alcohols can contain one or more hydroxyl group, and are thereby named depending upon the
number of hydroxyl group they possess, as mono, di, tri or polyhydric alcohols.
Aldehydes and ketones both contain C=O group, the carbonyl group. While ketones have the
carbonyl group joined on both ends with one ‘alkyl’ group, the aldehydes have one end attached
to alkyl group, and the other, with a hydrogen.
Example:
Hence, functional group of aldehydes is –CHO, while that of Ketones is C=O.
Chemistry 3
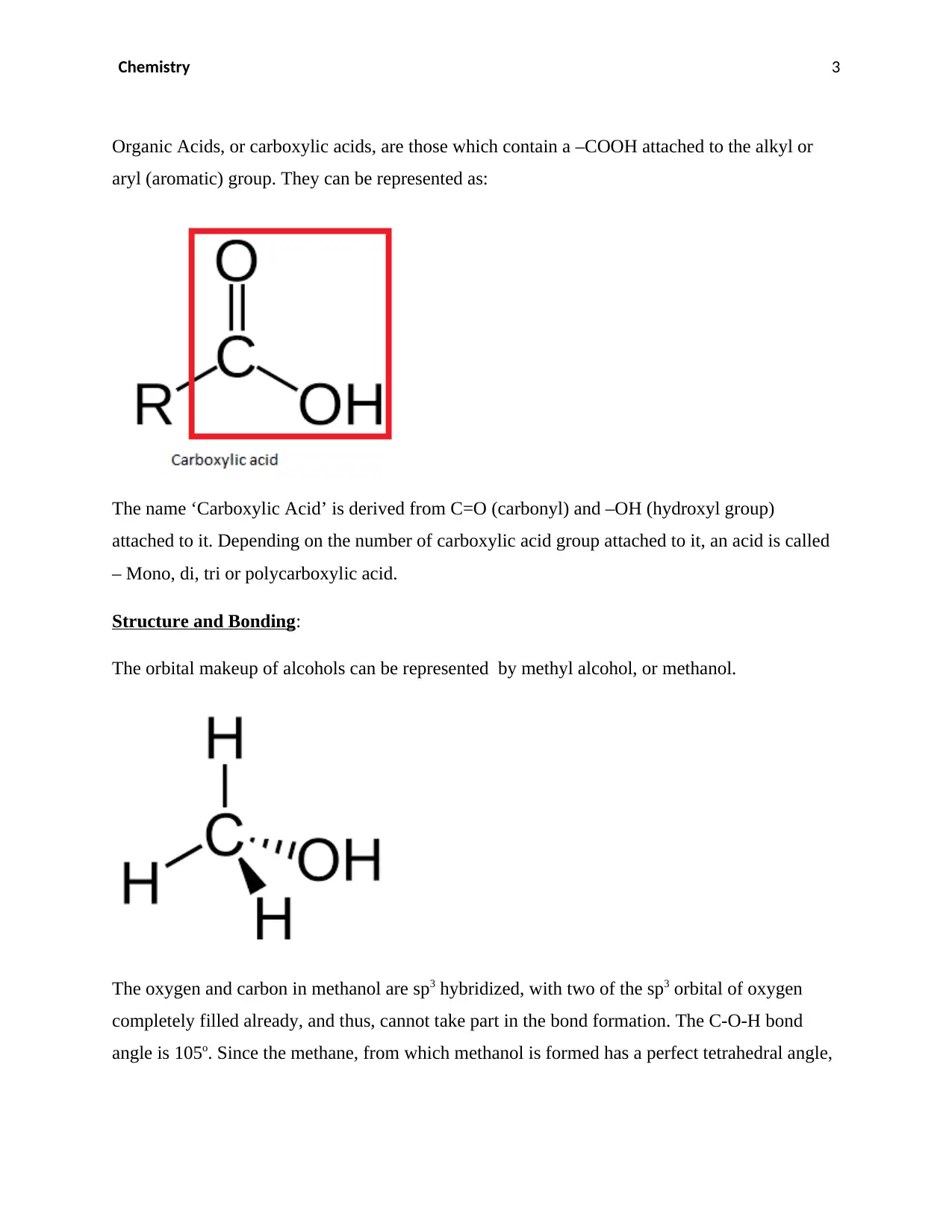
Organic Acids, or carboxylic acids, are those which contain a –COOH attached to the alkyl or
aryl (aromatic) group. They can be represented as:
The name ‘Carboxylic Acid’ is derived from C=O (carbonyl) and –OH (hydroxyl group)
attached to it. Depending on the number of carboxylic acid group attached to it, an acid is called
– Mono, di, tri or polycarboxylic acid.
Structure and Bonding:
The orbital makeup of alcohols can be represented by methyl alcohol, or methanol.
The oxygen and carbon in methanol are sp3 hybridized, with two of the sp3 orbital of oxygen
completely filled already, and thus, cannot take part in the bond formation. The C-O-H bond
angle is 105o. Since the methane, from which methanol is formed has a perfect tetrahedral angle,
Organic Acids, or carboxylic acids, are those which contain a –COOH attached to the alkyl or
aryl (aromatic) group. They can be represented as:
The name ‘Carboxylic Acid’ is derived from C=O (carbonyl) and –OH (hydroxyl group)
attached to it. Depending on the number of carboxylic acid group attached to it, an acid is called
– Mono, di, tri or polycarboxylic acid.
Structure and Bonding:
The orbital makeup of alcohols can be represented by methyl alcohol, or methanol.
The oxygen and carbon in methanol are sp3 hybridized, with two of the sp3 orbital of oxygen
completely filled already, and thus, cannot take part in the bond formation. The C-O-H bond
angle is 105o. Since the methane, from which methanol is formed has a perfect tetrahedral angle,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemistry 4
it is only proven that the bond angle is reduced in methanol due to the presence of the oxygen
atom. (and the filled sp3 orbital of oxygen repelling each other).
Aldehydes and ketones, with their double bond between carbon and oxygen (in C=O group), are
sp2 hybridized, since both carbon and oxygen are sp2 hybridized. The bond angle (with the three
atoms attached to central carbon lie on the same plane) is a perfect 120o.
Nomenclature:
The naming of an organic compound is usually done depending upon their ‘functional group’.
While the rules applied may be the same for some, the ‘counting of the number of carbon atoms’,
and the way an alkyl group is named (either first or second), all depends upon the general as well
as the rules applied to individual functional groups.
Alcohols – IUPAC nomenclature named alcohols as ‘Alkanols’. Hence, an ending ‘ol’ is
attached to the alkyl group present in an alcohols.
Example: CH3CH2OH has an ethyl group attached to hydroxyl, and hence is named as
‘ETHANOL’- Ethane + OL = Ethanol.
Ketones, on the other hand, have two alkyl groups on either side of ‘C=O’, and hence, the
IUPAC system adds up the carbon atom of the carbonyl group, while naming the ketone.
Example: CH3-CO-CH3 is named as ‘Propanone’ – Propane (for three carbons, one of each
methyl group and one of carbonyl group) + one = Propanone.
Aldehydes:
For aldehydes, the ‘e’ of the alkyl group is replaced by ‘-al’. The difference between alcohol and
aldehyde is that while alcohol has an –OL- at the end, aldehyde has an –AL- at the end.
However, while counting the carbon atom, the carbon on the –CHO is also counted.
Example: CH3CHO is called as ETHANAL (Ethane + AL)
Carboxylic Acids:
it is only proven that the bond angle is reduced in methanol due to the presence of the oxygen
atom. (and the filled sp3 orbital of oxygen repelling each other).
Aldehydes and ketones, with their double bond between carbon and oxygen (in C=O group), are
sp2 hybridized, since both carbon and oxygen are sp2 hybridized. The bond angle (with the three
atoms attached to central carbon lie on the same plane) is a perfect 120o.
Nomenclature:
The naming of an organic compound is usually done depending upon their ‘functional group’.
While the rules applied may be the same for some, the ‘counting of the number of carbon atoms’,
and the way an alkyl group is named (either first or second), all depends upon the general as well
as the rules applied to individual functional groups.
Alcohols – IUPAC nomenclature named alcohols as ‘Alkanols’. Hence, an ending ‘ol’ is
attached to the alkyl group present in an alcohols.
Example: CH3CH2OH has an ethyl group attached to hydroxyl, and hence is named as
‘ETHANOL’- Ethane + OL = Ethanol.
Ketones, on the other hand, have two alkyl groups on either side of ‘C=O’, and hence, the
IUPAC system adds up the carbon atom of the carbonyl group, while naming the ketone.
Example: CH3-CO-CH3 is named as ‘Propanone’ – Propane (for three carbons, one of each
methyl group and one of carbonyl group) + one = Propanone.
Aldehydes:
For aldehydes, the ‘e’ of the alkyl group is replaced by ‘-al’. The difference between alcohol and
aldehyde is that while alcohol has an –OL- at the end, aldehyde has an –AL- at the end.
However, while counting the carbon atom, the carbon on the –CHO is also counted.
Example: CH3CHO is called as ETHANAL (Ethane + AL)
Carboxylic Acids:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 5
An –OIC acid- is added to the final –e- of the alkyl group. (Here again, the number of carbon
includes the –COOH- carbon too.
Example: CH3COOH, commonly called acetic acid, is ETHANOIC ACID, since it consists of
two carbon atoms, signifying ETHANE.
Physical Properties:
a. In alcohols, the lower members are colorless, toxic liquids. They have a characteristic
odor, and their boiling points increase with increasing number of carbon atoms.
b. While the first member, formaldehyde is a gas, all other aldehydes are liquids at room
temperature, and have unpleasant odors.
c. The ketones, while being liquids at room temperature, have sweet smelling and
characteristic odor.
d. Acids are (lower members, upto C10) are unpleasant smelling liquids (example – vinegar,
acetic acid) and higher members are wax-like solids and are odorless.
Interconversions:
Alcohols, ketones, aldehydes and acids can be interconverted into one another. The below
equation gives an example of how one organic compound can be prepared from the other:
Secondary alcohols can also be oxidized to ketones. Oxidation reactions of alcohols, both
primary and secondary, to yield aldehydes, ketones and carboxylic acids are common practice
for their laboratory and large scale preparation.
Alternatively, reduction of aldehydes and ketones yields alcohols.
An –OIC acid- is added to the final –e- of the alkyl group. (Here again, the number of carbon
includes the –COOH- carbon too.
Example: CH3COOH, commonly called acetic acid, is ETHANOIC ACID, since it consists of
two carbon atoms, signifying ETHANE.
Physical Properties:
a. In alcohols, the lower members are colorless, toxic liquids. They have a characteristic
odor, and their boiling points increase with increasing number of carbon atoms.
b. While the first member, formaldehyde is a gas, all other aldehydes are liquids at room
temperature, and have unpleasant odors.
c. The ketones, while being liquids at room temperature, have sweet smelling and
characteristic odor.
d. Acids are (lower members, upto C10) are unpleasant smelling liquids (example – vinegar,
acetic acid) and higher members are wax-like solids and are odorless.
Interconversions:
Alcohols, ketones, aldehydes and acids can be interconverted into one another. The below
equation gives an example of how one organic compound can be prepared from the other:
Secondary alcohols can also be oxidized to ketones. Oxidation reactions of alcohols, both
primary and secondary, to yield aldehydes, ketones and carboxylic acids are common practice
for their laboratory and large scale preparation.
Alternatively, reduction of aldehydes and ketones yields alcohols.

Chemistry 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemistry 7
References
Aaaron, C., nd. What are alcohols? Alcohol, Carboxylic Acid and Esters, Available
at<https://sites.google.com/site/chemistryolp/introduction>[Accssesed 3 may 2017.]
Clark, J. 2003. Introducing Alcohols, Available
athttp://www.chemguide.co.uk/organicprops/alcohols/background.html>[Accssesed 3 may
2017].
References
Aaaron, C., nd. What are alcohols? Alcohol, Carboxylic Acid and Esters, Available
at<https://sites.google.com/site/chemistryolp/introduction>[Accssesed 3 may 2017.]
Clark, J. 2003. Introducing Alcohols, Available
athttp://www.chemguide.co.uk/organicprops/alcohols/background.html>[Accssesed 3 may
2017].
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





