CHEM 108: Experiment 2 Lab Report - Organic Compounds Analysis
VerifiedAdded on 2022/08/13
|4
|362
|30
Report
AI Summary
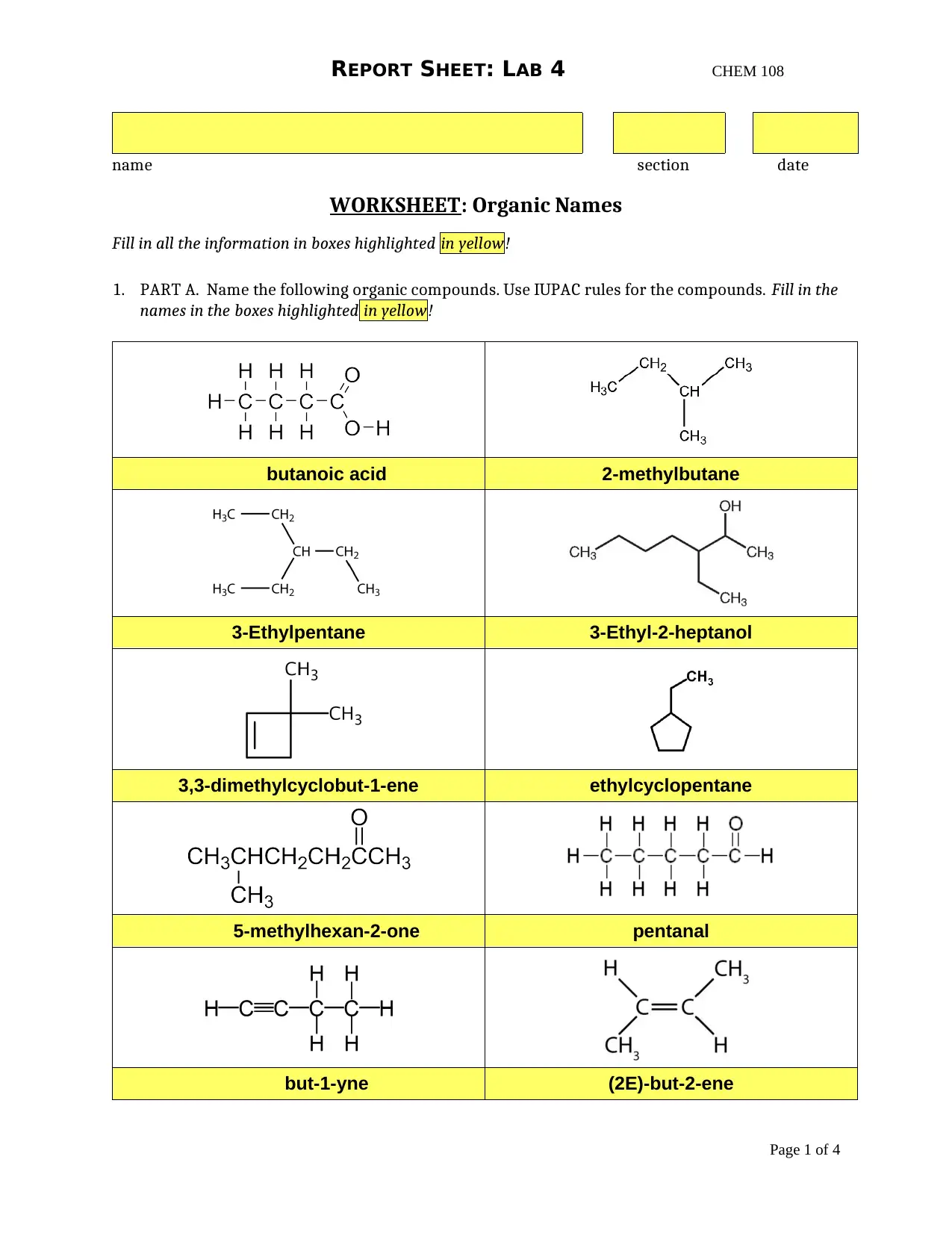
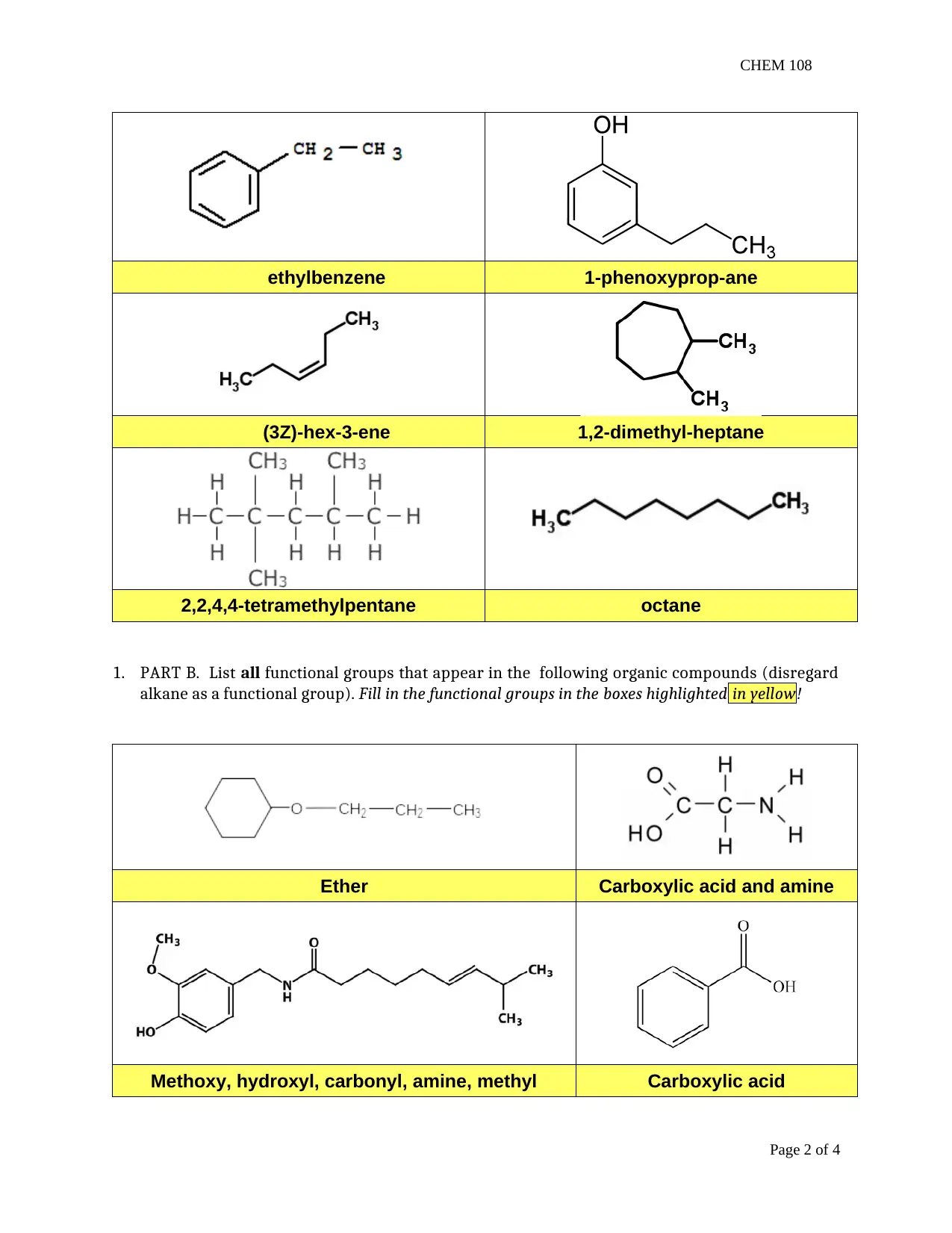
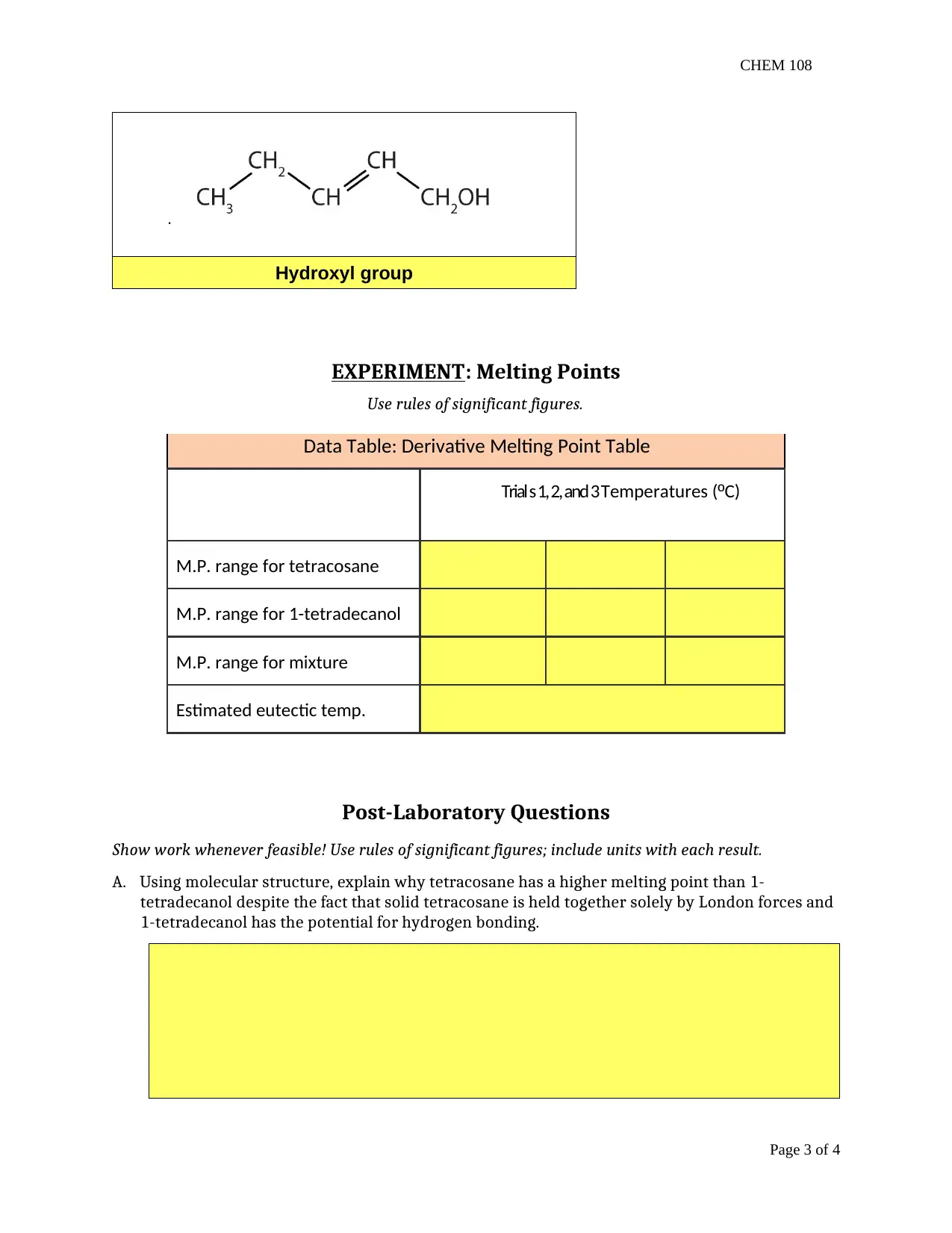
This report presents the findings of a chemistry lab experiment (CHEM 108) focusing on organic chemistry concepts. The assignment is divided into two main parts: organic compound naming and melting point determination. The first part involves naming various organic compounds based on IUPAC rules, including alkanes, alkenes, and compounds with functional groups. The second part details an experiment on melting points, where students determine the melting point ranges of tetracosane, 1-tetradecanol, and their mixture. The report includes data tables, observations, and post-laboratory questions that require students to analyze the relationship between molecular structure and melting point, the effects of heating rate on melting point accuracy, and the purity of substances based on melting point behavior. It also explores the functional groups present in the organic compounds and the significance of experimental procedures like using fresh water and fresh samples for accurate results. The assignment aims to reinforce the understanding of organic nomenclature, intermolecular forces, and experimental techniques in determining physical properties.
1 out of 4


![[object Object]](/_next/static/media/star-bottom.7253800d.svg)