Patients' Preferences Related to Schizophrenia Treatment: A Study
VerifiedAdded on 2023/05/29
|8
|6801
|376
Report
AI Summary
This study, published in Psychiatric Services in Advance, investigates patients' preferences regarding the benefits, risks, and formulations of antipsychotic treatments for schizophrenia. Using a discrete-choice experiment, the research quantified patient preferences by presenting hypothetical scenarios with varying improvements in positive, negative, and social functioning, along with the incidence of side effects such as weight gain, extrapyramidal symptoms, hyperprolactinemia, and hyperglycemia. The study, involving 271 patients, revealed that improvement in positive symptoms was the most preferred outcome, followed by avoiding hyperglycemia. Patients preferred oral formulations for adherent patients and monthly injections for nonadherent patients. The findings highlight the importance of considering patient preferences when making treatment decisions and balancing symptom alleviation with adverse event minimization, especially concerning medication adherence and formulation choices.

Patients’Preferences Related to Benefits, Risks, and
Formulations of Schizophrenia Treatment
Bennett Levitan,M.D.,Ph.D.,MichaelMarkowitz,M.D.,M.S.P.H.,Ateesha F.Mohamed,M.A.,F. Reed Johnson,Ph.D.,
Larry Alphs,M.D.,Ph.D.,Leslie Citrome,M.D.,M.P.H.,John F. P. Bridges,Ph.D.
Objective: The objective of this study was to quantify pa-
tients’preferences related to benefits and risks of antipsy-
chotic treatments for schizophrenia and to assess the relative
importance of treatment attributes and adherence.
Methods: Treatment-related preferences among U.S.res-
idents with a self-reported physician diagnosis of schizo-
phrenia were assessed via a discrete-choice experiment.
Patients chose between competing hypothetical scenarios
characterized by improvements in positive symptoms,neg-
ative symptoms, and social functioning; incidence of weight
gain, extrapyramidal symptoms (EPS), hyperprolactinemia, and
hyperglycemia; and medication formulation. Preferences were
estimated by using a random-parameters logit model, and the
impact of adherence was estimated with conditionallogit
models.
Results: The final sample consisted of 271 patients. Complete
improvement in positive symptoms was the most preferred
outcome (relative importance score of10.0),followed by
elimination of hyperglycemia (3.6,95% confidence interval
[CI]=2.6–4.6),improvement in negative symptoms (3.0,CI=
1.6–4.3), reduced weight gain (2.6, CI=1.2–4.0), avoidance
of hyperprolactinemia (1.7, CI=.9–2.6), improved social func-
tioning (1.5,CI=.4–2.5), and avoidance of EPS (1.0,CI=
.3–1.8). Patients judged a daily pill superior to monthly injections
(p,.01) and monthly injections superior to injections every three
months (p,.01) for adherent patients and monthly injections
superior to a daily pill for nonadherent patients (p=.01).
Conclusions: Persons who self-identified as having schizo-
phrenia judged improvement in positive symptoms as the
most important treatment benefit. Hyperglycemia was iden-
tified as the most important adverse event.Patients judged
oral formulations to be better than monthly injections for
adherent patients and monthly injections to be a better
choice for nonadherent patients.
Psychiatric Services in Advance, March 16, 2015; doi: 10.1176/appi.
ps.201400188
Schizophrenia is a major psychotic disorder, with symptoms
including changes in perception,feeling,behavior,judgment,
ideation,thought process,and motivation.Symptom mani-
festation is heterogeneous and variable over time (1).The
lifetime prevalence of schizophrenia is approximately 1 in
100 (1),and the incidence of schizophrenia in the United
States is 11.1 per 100,000 (2).
Treatment of schizophrenia with antipsychotics requires a
balance between alleviation of symptoms and minimization of
adverse events (3). Desired benefits include avoiding relapse
or hospitalization, amelioration of positive and negative symp-
toms, and improvement in psychosocial and occupational
functioning. Common risks include weight gain and metabolic
disturbances (hyperglycemia,diabetes,and hyperlipidemia),
extrapyramidal symptoms (EPS),and prolactin elevation.
Treatment formulation is also important,especially con-
sidering the potential of long-acting injectables (LAIs) to
simplify dosing regimens and improve outcomes among
patients with poor adherence to medication (4).
Numerous choices of antipsychotic medications with vari-
ous efficacy and side-effect profiles are available to the clini
When deciding among treatments, physicians must consider
both available evidence and the preferences of patients (5),
which can be quantified by using stated-preference methods
such as discrete-choice experiments (DCEs), also known as
conjoint analyses (6,7). Previous studies of antipsychotic pre
erence have demonstrated that patients, physicians, and fam
members collectively place greater importance on productiv
activity (work or school) compared with positive symptoms
or social functioning and place less importance on negative
symptoms and side effects (8,9). Medication side effects are
greater concern among patients and their families than amo
clinicians (10–14).
Our study built on this research by identifying key at-
tributes (benefits and risks) of antipsychotics and quantify-
ing the trade-offs considered by patients when balancing
attributes with formulation and adherence. Novel aspects of
our work included use of a structured benefit-risk framework
PS in Advance ps.psychiatryonline.org1
ARTICLES
Formulations of Schizophrenia Treatment
Bennett Levitan,M.D.,Ph.D.,MichaelMarkowitz,M.D.,M.S.P.H.,Ateesha F.Mohamed,M.A.,F. Reed Johnson,Ph.D.,
Larry Alphs,M.D.,Ph.D.,Leslie Citrome,M.D.,M.P.H.,John F. P. Bridges,Ph.D.
Objective: The objective of this study was to quantify pa-
tients’preferences related to benefits and risks of antipsy-
chotic treatments for schizophrenia and to assess the relative
importance of treatment attributes and adherence.
Methods: Treatment-related preferences among U.S.res-
idents with a self-reported physician diagnosis of schizo-
phrenia were assessed via a discrete-choice experiment.
Patients chose between competing hypothetical scenarios
characterized by improvements in positive symptoms,neg-
ative symptoms, and social functioning; incidence of weight
gain, extrapyramidal symptoms (EPS), hyperprolactinemia, and
hyperglycemia; and medication formulation. Preferences were
estimated by using a random-parameters logit model, and the
impact of adherence was estimated with conditionallogit
models.
Results: The final sample consisted of 271 patients. Complete
improvement in positive symptoms was the most preferred
outcome (relative importance score of10.0),followed by
elimination of hyperglycemia (3.6,95% confidence interval
[CI]=2.6–4.6),improvement in negative symptoms (3.0,CI=
1.6–4.3), reduced weight gain (2.6, CI=1.2–4.0), avoidance
of hyperprolactinemia (1.7, CI=.9–2.6), improved social func-
tioning (1.5,CI=.4–2.5), and avoidance of EPS (1.0,CI=
.3–1.8). Patients judged a daily pill superior to monthly injections
(p,.01) and monthly injections superior to injections every three
months (p,.01) for adherent patients and monthly injections
superior to a daily pill for nonadherent patients (p=.01).
Conclusions: Persons who self-identified as having schizo-
phrenia judged improvement in positive symptoms as the
most important treatment benefit. Hyperglycemia was iden-
tified as the most important adverse event.Patients judged
oral formulations to be better than monthly injections for
adherent patients and monthly injections to be a better
choice for nonadherent patients.
Psychiatric Services in Advance, March 16, 2015; doi: 10.1176/appi.
ps.201400188
Schizophrenia is a major psychotic disorder, with symptoms
including changes in perception,feeling,behavior,judgment,
ideation,thought process,and motivation.Symptom mani-
festation is heterogeneous and variable over time (1).The
lifetime prevalence of schizophrenia is approximately 1 in
100 (1),and the incidence of schizophrenia in the United
States is 11.1 per 100,000 (2).
Treatment of schizophrenia with antipsychotics requires a
balance between alleviation of symptoms and minimization of
adverse events (3). Desired benefits include avoiding relapse
or hospitalization, amelioration of positive and negative symp-
toms, and improvement in psychosocial and occupational
functioning. Common risks include weight gain and metabolic
disturbances (hyperglycemia,diabetes,and hyperlipidemia),
extrapyramidal symptoms (EPS),and prolactin elevation.
Treatment formulation is also important,especially con-
sidering the potential of long-acting injectables (LAIs) to
simplify dosing regimens and improve outcomes among
patients with poor adherence to medication (4).
Numerous choices of antipsychotic medications with vari-
ous efficacy and side-effect profiles are available to the clini
When deciding among treatments, physicians must consider
both available evidence and the preferences of patients (5),
which can be quantified by using stated-preference methods
such as discrete-choice experiments (DCEs), also known as
conjoint analyses (6,7). Previous studies of antipsychotic pre
erence have demonstrated that patients, physicians, and fam
members collectively place greater importance on productiv
activity (work or school) compared with positive symptoms
or social functioning and place less importance on negative
symptoms and side effects (8,9). Medication side effects are
greater concern among patients and their families than amo
clinicians (10–14).
Our study built on this research by identifying key at-
tributes (benefits and risks) of antipsychotics and quantify-
ing the trade-offs considered by patients when balancing
attributes with formulation and adherence. Novel aspects of
our work included use of a structured benefit-risk framework
PS in Advance ps.psychiatryonline.org1
ARTICLES
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

to identify a set of the most critical benefits and harms that
physicians consider in antipsychotic treatment decisions, a
quantitative approach to assess judgments for trade-offs be-
tween formulation and benefits, and a method for quantifying
the impact of adherence on these trade-offs.
METHODS
DCE Study
DCE studies quantify respondent preferences via a series
of choice tasks requiring respondents to indicate which of
severalhypotheticaltreatment alternatives they prefer.
Treatment alternatives are defined by systematically altering
various treatment attributes,exposing respondents to hypo-
theticaltreatments that are reminiscentof but not repre-
sentative of any existing treatment. DCEs are now common
(7,15–17) and are increasingly applied in online surveys (6,7,16).
Regression analysis of the association of respondents’ choices
and the attributes of the treatment alternatives allows for an
estimation of the relative importance of the attributes con-
sidered (6,18).
Study Sample
We targeted a sample of 300 patients, a sample size consis-
tent with both current DCE guidelines (19,20) and current
DCE practices in health (18). Respondents who were at least
18 years of age and who had a self-reported physician di-
agnosis of schizophrenia were identified via a prescreening
survey and were recruited through Kantar Health’s online
patient panel in May 2012. In total, 811 respondents received
e-mail invitations to participate in the online survey.Respon-
dents received “points” equivalent to 5–10 euros ($6–$13 U.S.)
that could be redeemed for merchandise or services or donated
to charity. The Office of Research Protection and Ethics at RTI
International approved this study.
Survey Instrument
This survey was developed in conjunction with a similar
instrument targeted to physicians (21,22). Applying an approach
based in multicriteria decision analysis and the Benefit-Risk
Action Team (BRAT) framework (23,24), we conducted written
and telephone interviews with expert academic psychiatrists
and assessed productinserts and publications to determine
what attributes to include in the survey. Seven attributes were
chosen, including improvements in three domains of symptoms
(positive symptoms, negative symptoms, and social function-
ing) and incidence of four adverse events (weight gain, EPS,
hyperprolactinemia [irregular periods or difficulty getting or
maintaining erections], and hyperglycemia). To assess validity
and reliability of our instrument, a draft version was tested in
12 open-ended cognitive interviews (25), after which minor
changes were made to the wording to improve respondent
comprehension.
Our survey used two types of choice tasks, one to assess
treatment preferences and another to assess the impact of
formulation and adherence. In the preference task, respondents
randomly received one of six blocks of eight randomly order
choice questions generated from a main-effects,D-optimal
experimental design consisting of 48 paired treatments (26,
Levels for positive and negative symptoms corresponded to
absent,mild,moderate,and severe levels in the Positive and
Negative Syndrome Scale (PANSS), with descriptions based
symptom lists from the PANSS scoring convention.The
choices consisted of a pair of treatments, each characterized
profiles of the seven attributes (Table 1); patients were aske
to choose which treatment was better for “Pat,” a hypothetic
patientwith schizophrenia.[An example ofa preference
choice task question is available in an online supplement to
this article.] Consistent with previous applications of DCEs
among patients with schizophrenia (10), respondents’pref-
erences were elicited by making a judgment about which
choice is better for a third party.
In the adherence task,respondents randomly received
one of nine blocks of four randomly ordered choice tasks
considering the trade-offbetween formulation (daily pill,
monthly injection, or injection every three months) and risk
of experiencing mild and severe positive symptoms (Table 2
Positive symptoms were assessed by using three distribution
of mild and severe symptoms (50%/50%, 40%/60%, and 20%
80%). In these tasks, the same treatments were compared f
both an adherent patient (“Pat”) and a patient who misses h
oral antipsychotic medications (“Jaime”).[An example of a
formulation and adherence choice task question is available
the online supplement.]
Statistical Analysis
Responses to the preference choice tasks were analyzed by
using a random-parameters logit model,in which a regres-
sion examined the association of respondents’choices and at-
tribute levels in each scenario, allowing estimation of the rel
importance of each attribute level (6,25,28). For the adheren
choice tasks, conditional logit models were used to estimate
relative importance weights for formulation and chance of im
provement in positive symptoms,given information regarding
patient adherence history. All analyses were conducted by u
NLOGIT 4.0.
RESULTS
Sample Characteristics
Of the 811 patients invited to participate, 684 (84%) respond
and 329 (41%) were eligible and provided informed consent
Of eligible respondents who consented, 301 (91%) answered
least one choice question.
Of these 301 respondents,30 chose the same response
(medicine A or B) for all eight preference choice questions.
Given the random assignment of attribute profiles into col-
umn A or B,this pattern should occur for only 2.4 respon-
dents, suggesting that these 30 respondents did not focus o
the survey (29). Data from these respondents were excluded
because of validity concerns,leaving 271 respondents in the
final sample.
2 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
physicians consider in antipsychotic treatment decisions, a
quantitative approach to assess judgments for trade-offs be-
tween formulation and benefits, and a method for quantifying
the impact of adherence on these trade-offs.
METHODS
DCE Study
DCE studies quantify respondent preferences via a series
of choice tasks requiring respondents to indicate which of
severalhypotheticaltreatment alternatives they prefer.
Treatment alternatives are defined by systematically altering
various treatment attributes,exposing respondents to hypo-
theticaltreatments that are reminiscentof but not repre-
sentative of any existing treatment. DCEs are now common
(7,15–17) and are increasingly applied in online surveys (6,7,16).
Regression analysis of the association of respondents’ choices
and the attributes of the treatment alternatives allows for an
estimation of the relative importance of the attributes con-
sidered (6,18).
Study Sample
We targeted a sample of 300 patients, a sample size consis-
tent with both current DCE guidelines (19,20) and current
DCE practices in health (18). Respondents who were at least
18 years of age and who had a self-reported physician di-
agnosis of schizophrenia were identified via a prescreening
survey and were recruited through Kantar Health’s online
patient panel in May 2012. In total, 811 respondents received
e-mail invitations to participate in the online survey.Respon-
dents received “points” equivalent to 5–10 euros ($6–$13 U.S.)
that could be redeemed for merchandise or services or donated
to charity. The Office of Research Protection and Ethics at RTI
International approved this study.
Survey Instrument
This survey was developed in conjunction with a similar
instrument targeted to physicians (21,22). Applying an approach
based in multicriteria decision analysis and the Benefit-Risk
Action Team (BRAT) framework (23,24), we conducted written
and telephone interviews with expert academic psychiatrists
and assessed productinserts and publications to determine
what attributes to include in the survey. Seven attributes were
chosen, including improvements in three domains of symptoms
(positive symptoms, negative symptoms, and social function-
ing) and incidence of four adverse events (weight gain, EPS,
hyperprolactinemia [irregular periods or difficulty getting or
maintaining erections], and hyperglycemia). To assess validity
and reliability of our instrument, a draft version was tested in
12 open-ended cognitive interviews (25), after which minor
changes were made to the wording to improve respondent
comprehension.
Our survey used two types of choice tasks, one to assess
treatment preferences and another to assess the impact of
formulation and adherence. In the preference task, respondents
randomly received one of six blocks of eight randomly order
choice questions generated from a main-effects,D-optimal
experimental design consisting of 48 paired treatments (26,
Levels for positive and negative symptoms corresponded to
absent,mild,moderate,and severe levels in the Positive and
Negative Syndrome Scale (PANSS), with descriptions based
symptom lists from the PANSS scoring convention.The
choices consisted of a pair of treatments, each characterized
profiles of the seven attributes (Table 1); patients were aske
to choose which treatment was better for “Pat,” a hypothetic
patientwith schizophrenia.[An example ofa preference
choice task question is available in an online supplement to
this article.] Consistent with previous applications of DCEs
among patients with schizophrenia (10), respondents’pref-
erences were elicited by making a judgment about which
choice is better for a third party.
In the adherence task,respondents randomly received
one of nine blocks of four randomly ordered choice tasks
considering the trade-offbetween formulation (daily pill,
monthly injection, or injection every three months) and risk
of experiencing mild and severe positive symptoms (Table 2
Positive symptoms were assessed by using three distribution
of mild and severe symptoms (50%/50%, 40%/60%, and 20%
80%). In these tasks, the same treatments were compared f
both an adherent patient (“Pat”) and a patient who misses h
oral antipsychotic medications (“Jaime”).[An example of a
formulation and adherence choice task question is available
the online supplement.]
Statistical Analysis
Responses to the preference choice tasks were analyzed by
using a random-parameters logit model,in which a regres-
sion examined the association of respondents’choices and at-
tribute levels in each scenario, allowing estimation of the rel
importance of each attribute level (6,25,28). For the adheren
choice tasks, conditional logit models were used to estimate
relative importance weights for formulation and chance of im
provement in positive symptoms,given information regarding
patient adherence history. All analyses were conducted by u
NLOGIT 4.0.
RESULTS
Sample Characteristics
Of the 811 patients invited to participate, 684 (84%) respond
and 329 (41%) were eligible and provided informed consent
Of eligible respondents who consented, 301 (91%) answered
least one choice question.
Of these 301 respondents,30 chose the same response
(medicine A or B) for all eight preference choice questions.
Given the random assignment of attribute profiles into col-
umn A or B,this pattern should occur for only 2.4 respon-
dents, suggesting that these 30 respondents did not focus o
the survey (29). Data from these respondents were excluded
because of validity concerns,leaving 271 respondents in the
final sample.
2 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
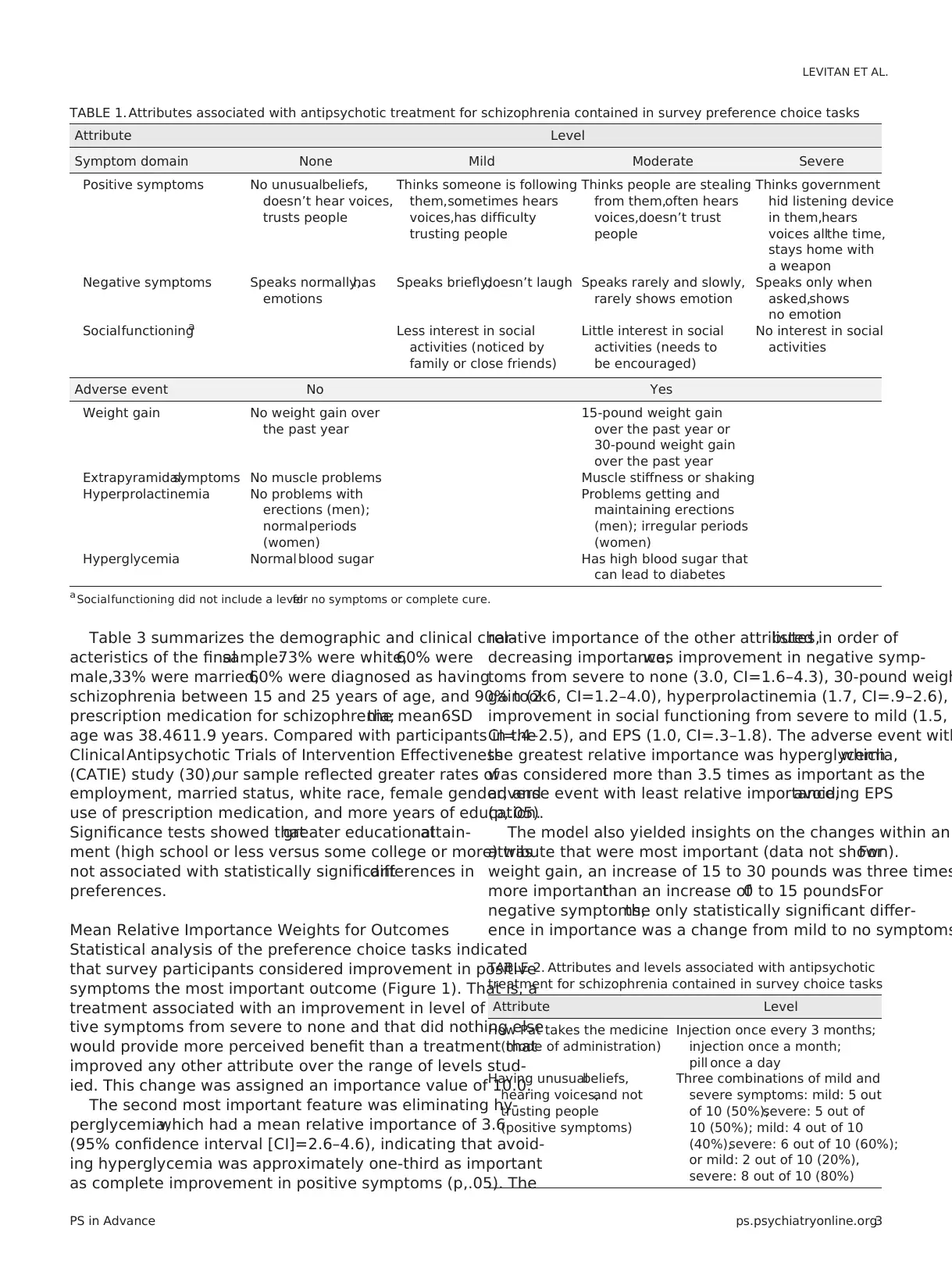
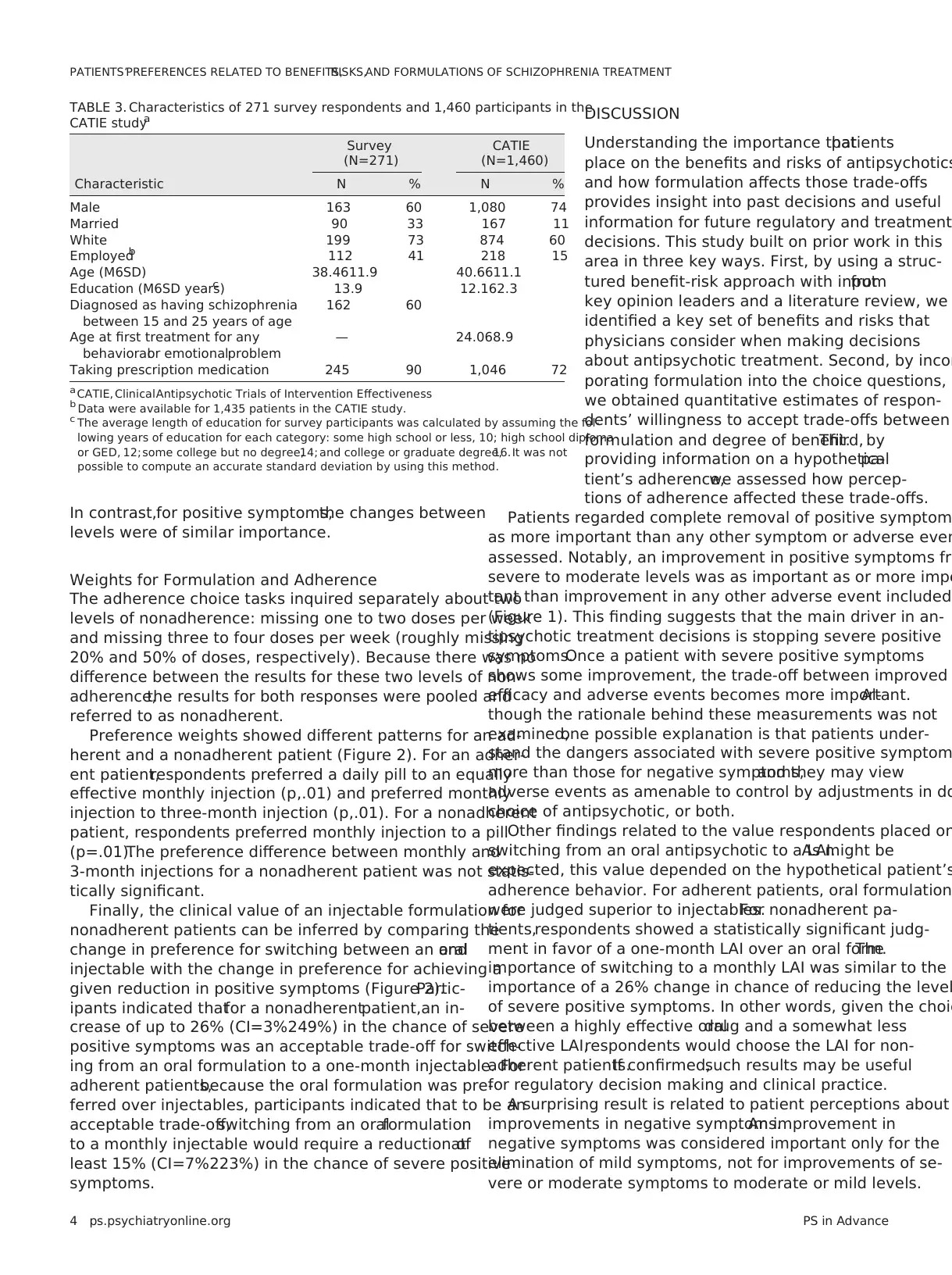
Table 3 summarizes the demographic and clinical char-
acteristics of the finalsample:73% were white,60% were
male,33% were married,60% were diagnosed as having
schizophrenia between 15 and 25 years of age, and 90% took
prescription medication for schizophrenia;the mean6SD
age was 38.4611.9 years. Compared with participants in the
ClinicalAntipsychotic Trials of Intervention Effectiveness
(CATIE) study (30),our sample reflected greater rates of
employment, married status, white race, female gender, and
use of prescription medication, and more years of education.
Significance tests showed thatgreater educationalattain-
ment (high school or less versus some college or more) was
not associated with statistically significantdifferences in
preferences.
Mean Relative Importance Weights for Outcomes
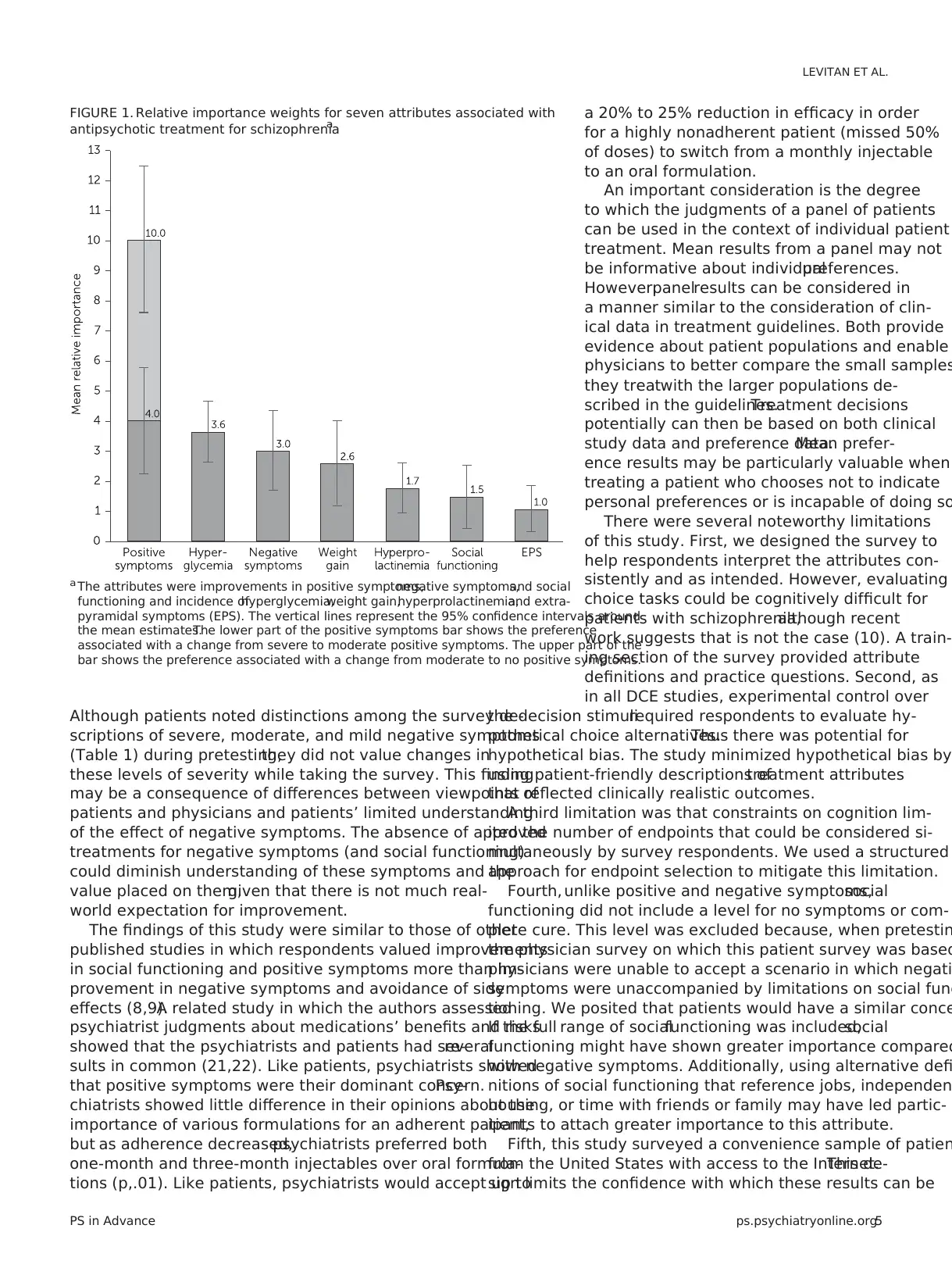
Statistical analysis of the preference choice tasks indicated
that survey participants considered improvement in positive
symptoms the most important outcome (Figure 1). That is, a
treatment associated with an improvement in level of posi-
tive symptoms from severe to none and that did nothing else
would provide more perceived benefit than a treatment that
improved any other attribute over the range of levels stud-
ied. This change was assigned an importance value of 10.0.
The second most important feature was eliminating hy-
perglycemia,which had a mean relative importance of 3.6
(95% confidence interval [CI]=2.6–4.6), indicating that avoid-
ing hyperglycemia was approximately one-third as important
as complete improvement in positive symptoms (p,.05). The
relative importance of the other attributes,listed in order of
decreasing importance,was improvement in negative symp-
toms from severe to none (3.0, CI=1.6–4.3), 30-pound weigh
gain (2.6, CI=1.2–4.0), hyperprolactinemia (1.7, CI=.9–2.6),
improvement in social functioning from severe to mild (1.5,
CI=.4–2.5), and EPS (1.0, CI=.3–1.8). The adverse event with
the greatest relative importance was hyperglycemia,which
was considered more than 3.5 times as important as the
adverse event with least relative importance,avoiding EPS
(p,.05).
The model also yielded insights on the changes within an
attribute that were most important (data not shown).For
weight gain, an increase of 15 to 30 pounds was three times
more importantthan an increase of0 to 15 pounds.For
negative symptoms,the only statistically significant differ-
ence in importance was a change from mild to no symptoms
TABLE 1. Attributes associated with antipsychotic treatment for schizophrenia contained in survey preference choice tasks
Attribute Level
Symptom domain None Mild Moderate Severe
Positive symptoms No unusualbeliefs,
doesn’t hear voices,
trusts people
Thinks someone is following
them,sometimes hears
voices,has difficulty
trusting people
Thinks people are stealing
from them,often hears
voices,doesn’t trust
people
Thinks government
hid listening device
in them,hears
voices allthe time,
stays home with
a weapon
Negative symptoms Speaks normally,has
emotions
Speaks briefly,doesn’t laugh Speaks rarely and slowly,
rarely shows emotion
Speaks only when
asked,shows
no emotion
Socialfunctioninga Less interest in social
activities (noticed by
family or close friends)
Little interest in social
activities (needs to
be encouraged)
No interest in social
activities
Adverse event No Yes
Weight gain No weight gain over
the past year
15-pound weight gain
over the past year or
30-pound weight gain
over the past year
Extrapyramidalsymptoms No muscle problems Muscle stiffness or shaking
Hyperprolactinemia No problems with
erections (men);
normalperiods
(women)
Problems getting and
maintaining erections
(men); irregular periods
(women)
Hyperglycemia Normal blood sugar Has high blood sugar that
can lead to diabetes
a Socialfunctioning did not include a levelfor no symptoms or complete cure.
TABLE 2. Attributes and levels associated with antipsychotic
treatment for schizophrenia contained in survey choice tasks
Attribute Level
How Pat takes the medicine
(mode of administration)
Injection once every 3 months;
injection once a month;
pill once a day
Having unusualbeliefs,
hearing voices,and not
trusting people
(positive symptoms)
Three combinations of mild and
severe symptoms: mild: 5 out
of 10 (50%),severe: 5 out of
10 (50%); mild: 4 out of 10
(40%),severe: 6 out of 10 (60%);
or mild: 2 out of 10 (20%),
severe: 8 out of 10 (80%)
PS in Advance ps.psychiatryonline.org3
LEVITAN ET AL.
acteristics of the finalsample:73% were white,60% were
male,33% were married,60% were diagnosed as having
schizophrenia between 15 and 25 years of age, and 90% took
prescription medication for schizophrenia;the mean6SD
age was 38.4611.9 years. Compared with participants in the
ClinicalAntipsychotic Trials of Intervention Effectiveness
(CATIE) study (30),our sample reflected greater rates of
employment, married status, white race, female gender, and
use of prescription medication, and more years of education.
Significance tests showed thatgreater educationalattain-
ment (high school or less versus some college or more) was
not associated with statistically significantdifferences in
preferences.
Mean Relative Importance Weights for Outcomes
Statistical analysis of the preference choice tasks indicated
that survey participants considered improvement in positive
symptoms the most important outcome (Figure 1). That is, a
treatment associated with an improvement in level of posi-
tive symptoms from severe to none and that did nothing else
would provide more perceived benefit than a treatment that
improved any other attribute over the range of levels stud-
ied. This change was assigned an importance value of 10.0.
The second most important feature was eliminating hy-
perglycemia,which had a mean relative importance of 3.6
(95% confidence interval [CI]=2.6–4.6), indicating that avoid-
ing hyperglycemia was approximately one-third as important
as complete improvement in positive symptoms (p,.05). The
relative importance of the other attributes,listed in order of
decreasing importance,was improvement in negative symp-
toms from severe to none (3.0, CI=1.6–4.3), 30-pound weigh
gain (2.6, CI=1.2–4.0), hyperprolactinemia (1.7, CI=.9–2.6),
improvement in social functioning from severe to mild (1.5,
CI=.4–2.5), and EPS (1.0, CI=.3–1.8). The adverse event with
the greatest relative importance was hyperglycemia,which
was considered more than 3.5 times as important as the
adverse event with least relative importance,avoiding EPS
(p,.05).
The model also yielded insights on the changes within an
attribute that were most important (data not shown).For
weight gain, an increase of 15 to 30 pounds was three times
more importantthan an increase of0 to 15 pounds.For
negative symptoms,the only statistically significant differ-
ence in importance was a change from mild to no symptoms
TABLE 1. Attributes associated with antipsychotic treatment for schizophrenia contained in survey preference choice tasks
Attribute Level
Symptom domain None Mild Moderate Severe
Positive symptoms No unusualbeliefs,
doesn’t hear voices,
trusts people
Thinks someone is following
them,sometimes hears
voices,has difficulty
trusting people
Thinks people are stealing
from them,often hears
voices,doesn’t trust
people
Thinks government
hid listening device
in them,hears
voices allthe time,
stays home with
a weapon
Negative symptoms Speaks normally,has
emotions
Speaks briefly,doesn’t laugh Speaks rarely and slowly,
rarely shows emotion
Speaks only when
asked,shows
no emotion
Socialfunctioninga Less interest in social
activities (noticed by
family or close friends)
Little interest in social
activities (needs to
be encouraged)
No interest in social
activities
Adverse event No Yes
Weight gain No weight gain over
the past year
15-pound weight gain
over the past year or
30-pound weight gain
over the past year
Extrapyramidalsymptoms No muscle problems Muscle stiffness or shaking
Hyperprolactinemia No problems with
erections (men);
normalperiods
(women)
Problems getting and
maintaining erections
(men); irregular periods
(women)
Hyperglycemia Normal blood sugar Has high blood sugar that
can lead to diabetes
a Socialfunctioning did not include a levelfor no symptoms or complete cure.
TABLE 2. Attributes and levels associated with antipsychotic
treatment for schizophrenia contained in survey choice tasks
Attribute Level
How Pat takes the medicine
(mode of administration)
Injection once every 3 months;
injection once a month;
pill once a day
Having unusualbeliefs,
hearing voices,and not
trusting people
(positive symptoms)
Three combinations of mild and
severe symptoms: mild: 5 out
of 10 (50%),severe: 5 out of
10 (50%); mild: 4 out of 10
(40%),severe: 6 out of 10 (60%);
or mild: 2 out of 10 (20%),
severe: 8 out of 10 (80%)
PS in Advance ps.psychiatryonline.org3
LEVITAN ET AL.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
In contrast,for positive symptoms,the changes between
levels were of similar importance.
Weights for Formulation and Adherence
The adherence choice tasks inquired separately about two
levels of nonadherence: missing one to two doses per week
and missing three to four doses per week (roughly missing
20% and 50% of doses, respectively). Because there was no
difference between the results for these two levels of non-
adherence,the results for both responses were pooled and
referred to as nonadherent.
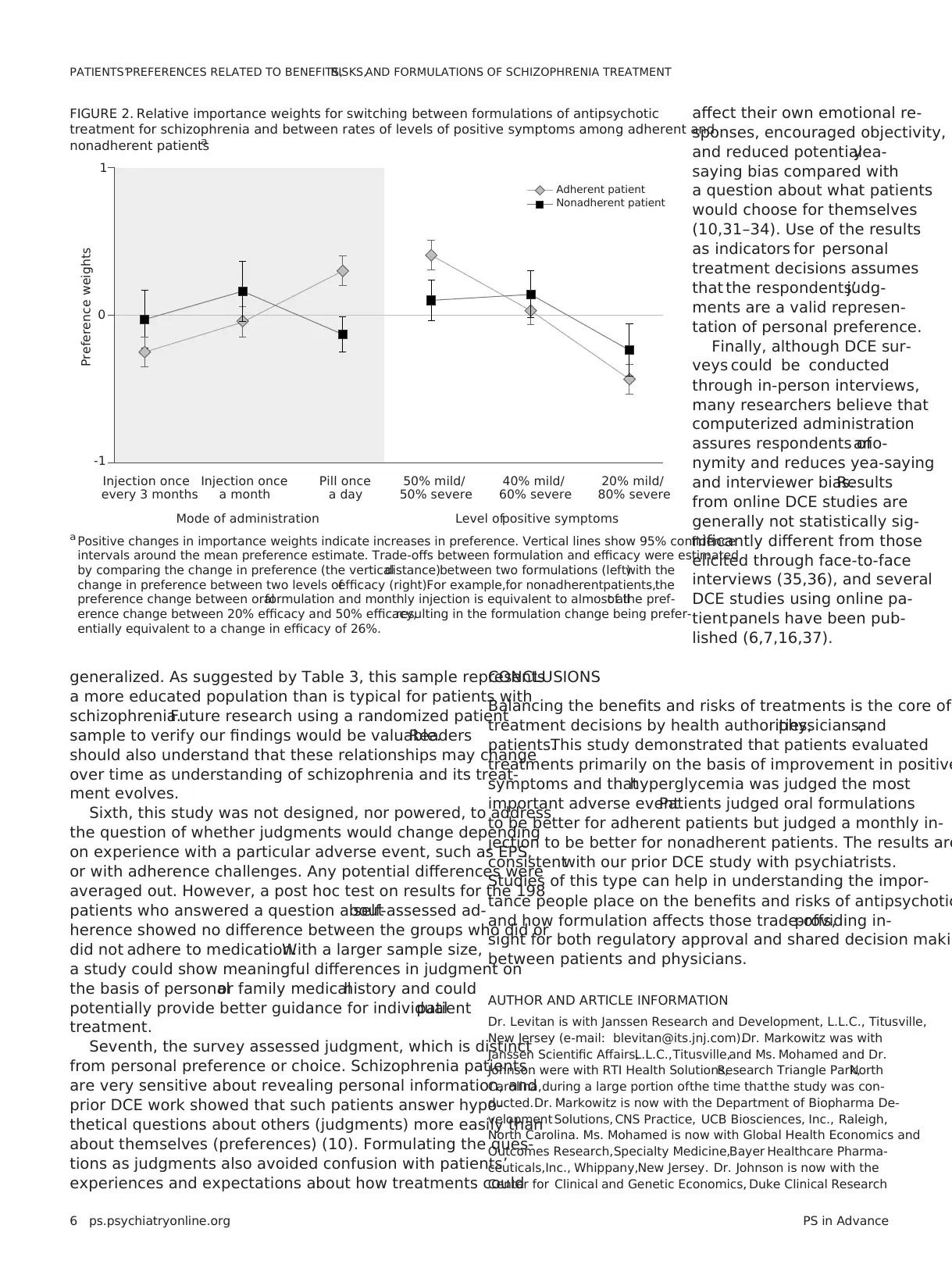
Preference weights showed different patterns for an ad-
herent and a nonadherent patient (Figure 2). For an adher-
ent patient,respondents preferred a daily pill to an equally
effective monthly injection (p,.01) and preferred monthly
injection to three-month injection (p,.01). For a nonadherent
patient, respondents preferred monthly injection to a pill
(p=.01).The preference difference between monthly and
3-month injections for a nonadherent patient was not statis-
tically significant.
Finally, the clinical value of an injectable formulation for
nonadherent patients can be inferred by comparing the
change in preference for switching between an oraland
injectable with the change in preference for achieving a
given reduction in positive symptoms (Figure 2).Partic-
ipants indicated thatfor a nonadherentpatient,an in-
crease of up to 26% (CI=3%249%) in the chance of severe
positive symptoms was an acceptable trade-off for switch-
ing from an oral formulation to a one-month injectable. For
adherent patients,because the oral formulation was pre-
ferred over injectables, participants indicated that to be an
acceptable trade-off,switching from an oralformulation
to a monthly injectable would require a reduction ofat
least 15% (CI=7%223%) in the chance of severe positive
symptoms.
DISCUSSION
Understanding the importance thatpatients
place on the benefits and risks of antipsychotics
and how formulation affects those trade-offs
provides insight into past decisions and useful
information for future regulatory and treatment
decisions. This study built on prior work in this
area in three key ways. First, by using a struc-
tured benefit-risk approach with inputfrom
key opinion leaders and a literature review, we
identified a key set of benefits and risks that
physicians consider when making decisions
about antipsychotic treatment. Second, by incor
porating formulation into the choice questions,
we obtained quantitative estimates of respon-
dents’ willingness to accept trade-offs between
formulation and degree of benefit.Third, by
providing information on a hypotheticalpa-
tient’s adherence,we assessed how percep-
tions of adherence affected these trade-offs.
Patients regarded complete removal of positive symptom
as more important than any other symptom or adverse even
assessed. Notably, an improvement in positive symptoms fro
severe to moderate levels was as important as or more impo
tant than improvement in any other adverse event included
(Figure 1). This finding suggests that the main driver in an-
tipsychotic treatment decisions is stopping severe positive
symptoms.Once a patient with severe positive symptoms
shows some improvement, the trade-off between improved
efficacy and adverse events becomes more important.Al-
though the rationale behind these measurements was not
examined,one possible explanation is that patients under-
stand the dangers associated with severe positive symptom
more than those for negative symptoms,and they may view
adverse events as amenable to control by adjustments in do
choice of antipsychotic, or both.
Other findings related to the value respondents placed on
switching from an oral antipsychotic to a LAI.As might be
expected, this value depended on the hypothetical patient’s
adherence behavior. For adherent patients, oral formulation
were judged superior to injectables.For nonadherent pa-
tients,respondents showed a statistically significant judg-
ment in favor of a one-month LAI over an oral form.The
importance of switching to a monthly LAI was similar to the
importance of a 26% change in chance of reducing the level
of severe positive symptoms. In other words, given the choic
between a highly effective oraldrug and a somewhat less
effective LAI,respondents would choose the LAI for non-
adherent patients.If confirmed,such results may be useful
for regulatory decision making and clinical practice.
A surprising result is related to patient perceptions about
improvements in negative symptoms.An improvement in
negative symptoms was considered important only for the
elimination of mild symptoms, not for improvements of se-
vere or moderate symptoms to moderate or mild levels.
TABLE 3. Characteristics of 271 survey respondents and 1,460 participants in the
CATIE studya
Survey
(N=271)
CATIE
(N=1,460)
Characteristic N % N %
Male 163 60 1,080 74
Married 90 33 167 11
White 199 73 874 60
Employedb 112 41 218 15
Age (M6SD) 38.4611.9 40.6611.1
Education (M6SD years)c 13.9 12.162.3
Diagnosed as having schizophrenia
between 15 and 25 years of age
162 60
Age at first treatment for any
behavioralor emotionalproblem
— 24.068.9
Taking prescription medication 245 90 1,046 72
a CATIE, ClinicalAntipsychotic Trials of Intervention Effectiveness
b Data were available for 1,435 patients in the CATIE study.
c The average length of education for survey participants was calculated by assuming the fol-
lowing years of education for each category: some high school or less, 10; high school diploma
or GED, 12; some college but no degree,14; and college or graduate degree,16. It was not
possible to compute an accurate standard deviation by using this method.
4 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
levels were of similar importance.
Weights for Formulation and Adherence
The adherence choice tasks inquired separately about two
levels of nonadherence: missing one to two doses per week
and missing three to four doses per week (roughly missing
20% and 50% of doses, respectively). Because there was no
difference between the results for these two levels of non-
adherence,the results for both responses were pooled and
referred to as nonadherent.
Preference weights showed different patterns for an ad-
herent and a nonadherent patient (Figure 2). For an adher-
ent patient,respondents preferred a daily pill to an equally
effective monthly injection (p,.01) and preferred monthly
injection to three-month injection (p,.01). For a nonadherent
patient, respondents preferred monthly injection to a pill
(p=.01).The preference difference between monthly and
3-month injections for a nonadherent patient was not statis-
tically significant.
Finally, the clinical value of an injectable formulation for
nonadherent patients can be inferred by comparing the
change in preference for switching between an oraland
injectable with the change in preference for achieving a
given reduction in positive symptoms (Figure 2).Partic-
ipants indicated thatfor a nonadherentpatient,an in-
crease of up to 26% (CI=3%249%) in the chance of severe
positive symptoms was an acceptable trade-off for switch-
ing from an oral formulation to a one-month injectable. For
adherent patients,because the oral formulation was pre-
ferred over injectables, participants indicated that to be an
acceptable trade-off,switching from an oralformulation
to a monthly injectable would require a reduction ofat
least 15% (CI=7%223%) in the chance of severe positive
symptoms.
DISCUSSION
Understanding the importance thatpatients
place on the benefits and risks of antipsychotics
and how formulation affects those trade-offs
provides insight into past decisions and useful
information for future regulatory and treatment
decisions. This study built on prior work in this
area in three key ways. First, by using a struc-
tured benefit-risk approach with inputfrom
key opinion leaders and a literature review, we
identified a key set of benefits and risks that
physicians consider when making decisions
about antipsychotic treatment. Second, by incor
porating formulation into the choice questions,
we obtained quantitative estimates of respon-
dents’ willingness to accept trade-offs between
formulation and degree of benefit.Third, by
providing information on a hypotheticalpa-
tient’s adherence,we assessed how percep-
tions of adherence affected these trade-offs.
Patients regarded complete removal of positive symptom
as more important than any other symptom or adverse even
assessed. Notably, an improvement in positive symptoms fro
severe to moderate levels was as important as or more impo
tant than improvement in any other adverse event included
(Figure 1). This finding suggests that the main driver in an-
tipsychotic treatment decisions is stopping severe positive
symptoms.Once a patient with severe positive symptoms
shows some improvement, the trade-off between improved
efficacy and adverse events becomes more important.Al-
though the rationale behind these measurements was not
examined,one possible explanation is that patients under-
stand the dangers associated with severe positive symptom
more than those for negative symptoms,and they may view
adverse events as amenable to control by adjustments in do
choice of antipsychotic, or both.
Other findings related to the value respondents placed on
switching from an oral antipsychotic to a LAI.As might be
expected, this value depended on the hypothetical patient’s
adherence behavior. For adherent patients, oral formulation
were judged superior to injectables.For nonadherent pa-
tients,respondents showed a statistically significant judg-
ment in favor of a one-month LAI over an oral form.The
importance of switching to a monthly LAI was similar to the
importance of a 26% change in chance of reducing the level
of severe positive symptoms. In other words, given the choic
between a highly effective oraldrug and a somewhat less
effective LAI,respondents would choose the LAI for non-
adherent patients.If confirmed,such results may be useful
for regulatory decision making and clinical practice.
A surprising result is related to patient perceptions about
improvements in negative symptoms.An improvement in
negative symptoms was considered important only for the
elimination of mild symptoms, not for improvements of se-
vere or moderate symptoms to moderate or mild levels.
TABLE 3. Characteristics of 271 survey respondents and 1,460 participants in the
CATIE studya
Survey
(N=271)
CATIE
(N=1,460)
Characteristic N % N %
Male 163 60 1,080 74
Married 90 33 167 11
White 199 73 874 60
Employedb 112 41 218 15
Age (M6SD) 38.4611.9 40.6611.1
Education (M6SD years)c 13.9 12.162.3
Diagnosed as having schizophrenia
between 15 and 25 years of age
162 60
Age at first treatment for any
behavioralor emotionalproblem
— 24.068.9
Taking prescription medication 245 90 1,046 72
a CATIE, ClinicalAntipsychotic Trials of Intervention Effectiveness
b Data were available for 1,435 patients in the CATIE study.
c The average length of education for survey participants was calculated by assuming the fol-
lowing years of education for each category: some high school or less, 10; high school diploma
or GED, 12; some college but no degree,14; and college or graduate degree,16. It was not
possible to compute an accurate standard deviation by using this method.
4 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Although patients noted distinctions among the survey de-
scriptions of severe, moderate, and mild negative symptoms
(Table 1) during pretesting,they did not value changes in
these levels of severity while taking the survey. This finding
may be a consequence of differences between viewpoints of
patients and physicians and patients’ limited understanding
of the effect of negative symptoms. The absence of approved
treatments for negative symptoms (and social functioning)
could diminish understanding of these symptoms and the
value placed on them,given that there is not much real-
world expectation for improvement.
The findings of this study were similar to those of other
published studies in which respondents valued improvements
in social functioning and positive symptoms more than im-
provement in negative symptoms and avoidance of side
effects (8,9).A related study in which the authors assessed
psychiatrist judgments about medications’ benefits and risks
showed that the psychiatrists and patients had severalre-
sults in common (21,22). Like patients, psychiatrists showed
that positive symptoms were their dominant concern.Psy-
chiatrists showed little difference in their opinions about the
importance of various formulations for an adherent patient,
but as adherence decreased,psychiatrists preferred both
one-month and three-month injectables over oral formula-
tions (p,.01). Like patients, psychiatrists would accept up to
a 20% to 25% reduction in efficacy in order
for a highly nonadherent patient (missed 50%
of doses) to switch from a monthly injectable
to an oral formulation.
An important consideration is the degree
to which the judgments of a panel of patients
can be used in the context of individual patient
treatment. Mean results from a panel may not
be informative about individualpreferences.
However,panelresults can be considered in
a manner similar to the consideration of clin-
ical data in treatment guidelines. Both provide
evidence about patient populations and enable
physicians to better compare the small samples
they treatwith the larger populations de-
scribed in the guidelines.Treatment decisions
potentially can then be based on both clinical
study data and preference data.Mean prefer-
ence results may be particularly valuable when
treating a patient who chooses not to indicate
personal preferences or is incapable of doing so
There were several noteworthy limitations
of this study. First, we designed the survey to
help respondents interpret the attributes con-
sistently and as intended. However, evaluating
choice tasks could be cognitively difficult for
patients with schizophrenia,although recent
work suggests that is not the case (10). A train-
ing section of the survey provided attribute
definitions and practice questions. Second, as
in all DCE studies, experimental control over
the decision stimulirequired respondents to evaluate hy-
pothetical choice alternatives.Thus there was potential for
hypothetical bias. The study minimized hypothetical bias by
using patient-friendly descriptions oftreatment attributes
that reflected clinically realistic outcomes.
A third limitation was that constraints on cognition lim-
ited the number of endpoints that could be considered si-
multaneously by survey respondents. We used a structured
approach for endpoint selection to mitigate this limitation.
Fourth, unlike positive and negative symptoms,social
functioning did not include a level for no symptoms or com-
plete cure. This level was excluded because, when pretestin
the physician survey on which this patient survey was based
physicians were unable to accept a scenario in which negati
symptoms were unaccompanied by limitations on social func
tioning. We posited that patients would have a similar conce
If the full range of socialfunctioning was included,social
functioning might have shown greater importance compared
with negative symptoms. Additionally, using alternative defi
nitions of social functioning that reference jobs, independen
housing, or time with friends or family may have led partic-
ipants to attach greater importance to this attribute.
Fifth, this study surveyed a convenience sample of patien
from the United States with access to the Internet.This de-
sign limits the confidence with which these results can be
FIGURE 1. Relative importance weights for seven attributes associated with
antipsychotic treatment for schizophreniaa
a The attributes were improvements in positive symptoms,negative symptoms,and social
functioning and incidence ofhyperglycemia,weight gain,hyperprolactinemia,and extra-
pyramidal symptoms (EPS). The vertical lines represent the 95% confidence intervals around
the mean estimates.The lower part of the positive symptoms bar shows the preference
associated with a change from severe to moderate positive symptoms. The upper part of the
bar shows the preference associated with a change from moderate to no positive symptoms.
PS in Advance ps.psychiatryonline.org5
LEVITAN ET AL.
scriptions of severe, moderate, and mild negative symptoms
(Table 1) during pretesting,they did not value changes in
these levels of severity while taking the survey. This finding
may be a consequence of differences between viewpoints of
patients and physicians and patients’ limited understanding
of the effect of negative symptoms. The absence of approved
treatments for negative symptoms (and social functioning)
could diminish understanding of these symptoms and the
value placed on them,given that there is not much real-
world expectation for improvement.
The findings of this study were similar to those of other
published studies in which respondents valued improvements
in social functioning and positive symptoms more than im-
provement in negative symptoms and avoidance of side
effects (8,9).A related study in which the authors assessed
psychiatrist judgments about medications’ benefits and risks
showed that the psychiatrists and patients had severalre-
sults in common (21,22). Like patients, psychiatrists showed
that positive symptoms were their dominant concern.Psy-
chiatrists showed little difference in their opinions about the
importance of various formulations for an adherent patient,
but as adherence decreased,psychiatrists preferred both
one-month and three-month injectables over oral formula-
tions (p,.01). Like patients, psychiatrists would accept up to
a 20% to 25% reduction in efficacy in order
for a highly nonadherent patient (missed 50%
of doses) to switch from a monthly injectable
to an oral formulation.
An important consideration is the degree
to which the judgments of a panel of patients
can be used in the context of individual patient
treatment. Mean results from a panel may not
be informative about individualpreferences.
However,panelresults can be considered in
a manner similar to the consideration of clin-
ical data in treatment guidelines. Both provide
evidence about patient populations and enable
physicians to better compare the small samples
they treatwith the larger populations de-
scribed in the guidelines.Treatment decisions
potentially can then be based on both clinical
study data and preference data.Mean prefer-
ence results may be particularly valuable when
treating a patient who chooses not to indicate
personal preferences or is incapable of doing so
There were several noteworthy limitations
of this study. First, we designed the survey to
help respondents interpret the attributes con-
sistently and as intended. However, evaluating
choice tasks could be cognitively difficult for
patients with schizophrenia,although recent
work suggests that is not the case (10). A train-
ing section of the survey provided attribute
definitions and practice questions. Second, as
in all DCE studies, experimental control over
the decision stimulirequired respondents to evaluate hy-
pothetical choice alternatives.Thus there was potential for
hypothetical bias. The study minimized hypothetical bias by
using patient-friendly descriptions oftreatment attributes
that reflected clinically realistic outcomes.
A third limitation was that constraints on cognition lim-
ited the number of endpoints that could be considered si-
multaneously by survey respondents. We used a structured
approach for endpoint selection to mitigate this limitation.
Fourth, unlike positive and negative symptoms,social
functioning did not include a level for no symptoms or com-
plete cure. This level was excluded because, when pretestin
the physician survey on which this patient survey was based
physicians were unable to accept a scenario in which negati
symptoms were unaccompanied by limitations on social func
tioning. We posited that patients would have a similar conce
If the full range of socialfunctioning was included,social
functioning might have shown greater importance compared
with negative symptoms. Additionally, using alternative defi
nitions of social functioning that reference jobs, independen
housing, or time with friends or family may have led partic-
ipants to attach greater importance to this attribute.
Fifth, this study surveyed a convenience sample of patien
from the United States with access to the Internet.This de-
sign limits the confidence with which these results can be
FIGURE 1. Relative importance weights for seven attributes associated with
antipsychotic treatment for schizophreniaa
a The attributes were improvements in positive symptoms,negative symptoms,and social
functioning and incidence ofhyperglycemia,weight gain,hyperprolactinemia,and extra-
pyramidal symptoms (EPS). The vertical lines represent the 95% confidence intervals around
the mean estimates.The lower part of the positive symptoms bar shows the preference
associated with a change from severe to moderate positive symptoms. The upper part of the
bar shows the preference associated with a change from moderate to no positive symptoms.
PS in Advance ps.psychiatryonline.org5
LEVITAN ET AL.
generalized. As suggested by Table 3, this sample represents
a more educated population than is typical for patients with
schizophrenia.Future research using a randomized patient
sample to verify our findings would be valuable.Readers
should also understand that these relationships may change
over time as understanding of schizophrenia and its treat-
ment evolves.
Sixth, this study was not designed, nor powered, to address
the question of whether judgments would change depending
on experience with a particular adverse event, such as EPS,
or with adherence challenges. Any potential differences were
averaged out. However, a post hoc test on results for the 198
patients who answered a question aboutself-assessed ad-
herence showed no difference between the groups who did or
did not adhere to medication.With a larger sample size,
a study could show meaningful differences in judgment on
the basis of personalor family medicalhistory and could
potentially provide better guidance for individualpatient
treatment.
Seventh, the survey assessed judgment, which is distinct
from personal preference or choice. Schizophrenia patients
are very sensitive about revealing personal information, and
prior DCE work showed that such patients answer hypo-
thetical questions about others (judgments) more easily than
about themselves (preferences) (10). Formulating the ques-
tions as judgments also avoided confusion with patients’
experiences and expectations about how treatments could
affect their own emotional re-
sponses, encouraged objectivity,
and reduced potentialyea-
saying bias compared with
a question about what patients
would choose for themselves
(10,31–34). Use of the results
as indicators for personal
treatment decisions assumes
that the respondents’judg-
ments are a valid represen-
tation of personal preference.
Finally, although DCE sur-
veys could be conducted
through in-person interviews,
many researchers believe that
computerized administration
assures respondents ofano-
nymity and reduces yea-saying
and interviewer bias.Results
from online DCE studies are
generally not statistically sig-
nificantly different from those
elicited through face-to-face
interviews (35,36), and several
DCE studies using online pa-
tientpanels have been pub-
lished (6,7,16,37).
CONCLUSIONS
Balancing the benefits and risks of treatments is the core of
treatment decisions by health authorities,physicians,and
patients.This study demonstrated that patients evaluated
treatments primarily on the basis of improvement in positive
symptoms and thathyperglycemia was judged the most
important adverse event.Patients judged oral formulations
to be better for adherent patients but judged a monthly in-
jection to be better for nonadherent patients. The results are
consistentwith our prior DCE study with psychiatrists.
Studies of this type can help in understanding the impor-
tance people place on the benefits and risks of antipsychotic
and how formulation affects those trade-offs,providing in-
sight for both regulatory approval and shared decision makin
between patients and physicians.
AUTHOR AND ARTICLE INFORMATION
Dr. Levitan is with Janssen Research and Development, L.L.C., Titusville,
New Jersey (e-mail: blevitan@its.jnj.com).Dr. Markowitz was with
Janssen Scientific Affairs,L.L.C.,Titusville,and Ms. Mohamed and Dr.
Johnson were with RTI Health Solutions,Research Triangle Park,North
Carolina,during a large portion ofthe time thatthe study was con-
ducted.Dr. Markowitz is now with the Department of Biopharma De-
velopment Solutions, CNS Practice, UCB Biosciences, Inc., Raleigh,
North Carolina. Ms. Mohamed is now with Global Health Economics and
Outcomes Research,Specialty Medicine,Bayer Healthcare Pharma-
ceuticals,Inc., Whippany,New Jersey. Dr. Johnson is now with the
Center for Clinical and Genetic Economics, Duke Clinical Research
FIGURE 2. Relative importance weights for switching between formulations of antipsychotic
treatment for schizophrenia and between rates of levels of positive symptoms among adherent and
nonadherent patientsa
1
-1
0
Preference weights
Adherent patient
Nonadherent patient
Injection once
every 3 months
Injection once
a month
Pill once
a day
50% mild/
50% severe
40% mild/
60% severe
20% mild/
80% severe
Mode of administration Level ofpositive symptoms
a Positive changes in importance weights indicate increases in preference. Vertical lines show 95% confidence
intervals around the mean preference estimate. Trade-offs between formulation and efficacy were estimated
by comparing the change in preference (the verticaldistance)between two formulations (left)with the
change in preference between two levels ofefficacy (right).For example,for nonadherentpatients,the
preference change between oralformulation and monthly injection is equivalent to almost allof the pref-
erence change between 20% efficacy and 50% efficacy,resulting in the formulation change being prefer-
entially equivalent to a change in efficacy of 26%.
6 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
a more educated population than is typical for patients with
schizophrenia.Future research using a randomized patient
sample to verify our findings would be valuable.Readers
should also understand that these relationships may change
over time as understanding of schizophrenia and its treat-
ment evolves.
Sixth, this study was not designed, nor powered, to address
the question of whether judgments would change depending
on experience with a particular adverse event, such as EPS,
or with adherence challenges. Any potential differences were
averaged out. However, a post hoc test on results for the 198
patients who answered a question aboutself-assessed ad-
herence showed no difference between the groups who did or
did not adhere to medication.With a larger sample size,
a study could show meaningful differences in judgment on
the basis of personalor family medicalhistory and could
potentially provide better guidance for individualpatient
treatment.
Seventh, the survey assessed judgment, which is distinct
from personal preference or choice. Schizophrenia patients
are very sensitive about revealing personal information, and
prior DCE work showed that such patients answer hypo-
thetical questions about others (judgments) more easily than
about themselves (preferences) (10). Formulating the ques-
tions as judgments also avoided confusion with patients’
experiences and expectations about how treatments could
affect their own emotional re-
sponses, encouraged objectivity,
and reduced potentialyea-
saying bias compared with
a question about what patients
would choose for themselves
(10,31–34). Use of the results
as indicators for personal
treatment decisions assumes
that the respondents’judg-
ments are a valid represen-
tation of personal preference.
Finally, although DCE sur-
veys could be conducted
through in-person interviews,
many researchers believe that
computerized administration
assures respondents ofano-
nymity and reduces yea-saying
and interviewer bias.Results
from online DCE studies are
generally not statistically sig-
nificantly different from those
elicited through face-to-face
interviews (35,36), and several
DCE studies using online pa-
tientpanels have been pub-
lished (6,7,16,37).
CONCLUSIONS
Balancing the benefits and risks of treatments is the core of
treatment decisions by health authorities,physicians,and
patients.This study demonstrated that patients evaluated
treatments primarily on the basis of improvement in positive
symptoms and thathyperglycemia was judged the most
important adverse event.Patients judged oral formulations
to be better for adherent patients but judged a monthly in-
jection to be better for nonadherent patients. The results are
consistentwith our prior DCE study with psychiatrists.
Studies of this type can help in understanding the impor-
tance people place on the benefits and risks of antipsychotic
and how formulation affects those trade-offs,providing in-
sight for both regulatory approval and shared decision makin
between patients and physicians.
AUTHOR AND ARTICLE INFORMATION
Dr. Levitan is with Janssen Research and Development, L.L.C., Titusville,
New Jersey (e-mail: blevitan@its.jnj.com).Dr. Markowitz was with
Janssen Scientific Affairs,L.L.C.,Titusville,and Ms. Mohamed and Dr.
Johnson were with RTI Health Solutions,Research Triangle Park,North
Carolina,during a large portion ofthe time thatthe study was con-
ducted.Dr. Markowitz is now with the Department of Biopharma De-
velopment Solutions, CNS Practice, UCB Biosciences, Inc., Raleigh,
North Carolina. Ms. Mohamed is now with Global Health Economics and
Outcomes Research,Specialty Medicine,Bayer Healthcare Pharma-
ceuticals,Inc., Whippany,New Jersey. Dr. Johnson is now with the
Center for Clinical and Genetic Economics, Duke Clinical Research
FIGURE 2. Relative importance weights for switching between formulations of antipsychotic
treatment for schizophrenia and between rates of levels of positive symptoms among adherent and
nonadherent patientsa
1
-1
0
Preference weights
Adherent patient
Nonadherent patient
Injection once
every 3 months
Injection once
a month
Pill once
a day
50% mild/
50% severe
40% mild/
60% severe
20% mild/
80% severe
Mode of administration Level ofpositive symptoms
a Positive changes in importance weights indicate increases in preference. Vertical lines show 95% confidence
intervals around the mean preference estimate. Trade-offs between formulation and efficacy were estimated
by comparing the change in preference (the verticaldistance)between two formulations (left)with the
change in preference between two levels ofefficacy (right).For example,for nonadherentpatients,the
preference change between oralformulation and monthly injection is equivalent to almost allof the pref-
erence change between 20% efficacy and 50% efficacy,resulting in the formulation change being prefer-
entially equivalent to a change in efficacy of 26%.
6 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Institute,Durham,North Carolina.Dr. Alphs is with Janssen Scientific
Affairs,L.L.C. Dr. Citrome is with the Departmentof Psychiatry and
BehavioralSciences,New York MedicalCollege,Valhalla.Dr. Bridges is
with the Department of Health Policy and Management, Johns Hopkins
Bloomberg Schoolof Public Health,Baltimore.
Funding for this study was obtained from Janssen Scientific Affairs, L.L.C.
The authors thank Vikram Kilambi, M.S., Angelyn Fairchild, B.A., and Gail
Zona, B.A., for their assistance in this project.The views expressed
herein do not necessarily reflect those ofJanssen Research and De-
velopment,L.L.C.,or Janssen Scientific Affairs,L.L.C.
Dr. Levitan is a stockholderin Baxter International,Inc., Johnson &
Johnson, Pharmaceutical HOLDRS Trust, and Zimmer Holdings, Inc. Dr.
Markowitz owns stock in GlaxoSmithKline,Johnson & Johnson, Pfizer,
and UCB.Ms. Mohamed holds stock in Bayer Healthcare Pharmaceut-
icals, Inc. Dr. Alphs holds stock in Johnson & Johnson. Dr. Citrome
serves as a consultant to or speaker for,or owns stock in, Actavis
(Forest),Alexza,Alkermes,AstraZeneca,Bristol-Myers Squibb,Eli Lilly,
Forum (Envivo),Genentech,Janssen, Jazz, Johnson & Johnson, Lund-
beck, Merck, Medivation, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt
Benckiser, Reviva, Shire, Sunovion, Takeda, and Teva. The other authors
report no financialrelationships with commercialinterests.
Received May 1,2014; revision received October 15,2014; accepted
December 4,2014; published online March 16,2015.
REFERENCES
1. Lehman AF, Lieberman JA, Dixon LB, et al: Practice guideline for
the treatment ofpatients with schizophrenia,2nd ed.American
Journal of Psychiatry 161(Feb suppl):1–56,2004
2. Goldner EM,Hsu L, Waraich P,et al:Prevalence and incidence
studies of schizophrenic disorders:a systematic review of the lit-
erature.Canadian Journal of Psychiatry 47:833–843,2002
3. Volavka J, Citrome L: Oral antipsychotics for the treatmentof
schizophrenia:heterogeneity in efficacy and tolerability should
drive decision-making.Expert Opinion on Pharmacotherapy 10:
1917–1928,2009
4. Citrome L: New second-generation long-acting injectable anti-
psychotics for the treatment of schizophrenia.Expert Review of
Neurotherapeutics 13:767–783,2013
5. Citrome L:Evidence-based medicine:it’s not just about the evi-
dence.International Journal of Clinical Practice 65:634–635,2011
6. Hauber AB,Arden NK,Mohamed AF,et al:A discrete-choice ex-
periment of United Kingdom patients’willingness to risk adverse
events for improved function and pain controlin osteoarthritis.
Osteoarthritis and Cartilage 21:289–297,2013
7. Bridges JF, Mohamed AF, Finnern HW, et al: Patients’ preferences
for treatment outcomes for advanced non-smallcell lung cancer:
a conjoint analysis.Lung Cancer 77:224–231,2012
8. Shumway M,Saunders T,Shern D,et al:Preferences for schizo-
phrenia treatmentoutcomes among public policy makers,con-
sumers, families, and providers. Psychiatric Services 54:1124–1128,
2003
9. Shumway M,Sentell T,Chouljian T,et al:Assessing preferences
for schizophrenia outcomes:comprehension and decision strate-
gies in three assessment methods. Mental Health Services Research
5:121–135,2003
10.Bridges JF, Kinter ET, Schmeding A, et al: Can patients diagnosed
with schizophrenia complete choice-based conjoint analysis tasks?
Patient 4:267–275,2011
11.Bridges JF, Slawik L, Schmeding A,et al:A test of concordance
between patient and psychiatrist valuations of multiple treatment
goals for schizophrenia.Health Expectations 16:164–176,2013
12.Kuhnigk O,Slawik L,Meyer J, et al:Valuation and attainment of
treatmentgoals in schizophrenia:perspectives ofpatients,rela-
tives, physicians, and payers. Journal of Psychiatric Practice 18:321–
328,2012
13.Shumway M: Preference weightsfor cost-outcome analysesof
schizophrenia treatments: comparison of four stakeholder groups.
Schizophrenia Bulletin 29:257–266,2003
14.Shumway M,Chouljian TL,Battle CL:Measuring preferences for
schizophrenia outcomes with the time tradeoff method. Journal of
Behavioral Health Services and Research 32:14–26,2005
15.Bryan S,Buxton M,Sheldon R,et al: Magnetic resonance imaging
for the investigation ofknee injuries:an investigation ofprefer-
ences.Health Economics 7:595–603,1998
16.Johnson FR, Ozdemir S,Manjunath R,et al: Factors that affect
adherence to bipolar disorder treatments:a stated-preference ap-
proach.Medical Care 45:545–552,2007
17.Clark MD, Determann D,Petrou S,et al:Discrete choice experi-
ments in health economics:a review of the literature.Pharmaco-
Economics 32:883–902,2014
18.MarshallD, Bridges JF,Hauber B,et al:Conjoint analysis appli-
cations in health—how are studies being designed and reported?
An update on current practice in the published literature between
2005 and 2008.Patient 3:249–256,2010
19.Johnson FR, Yang J-C, Mohamed AF:In defense ofimperfect
experimental designs; statistical efficiency and measurement error
in choice-format conjoint analysis. Presented at the Proceedings of
the Sawtooth Software Conference,Orlando,Fla, March 21–23,
2012
20. Louviere J, Swait J, Hensher D:Introduction to stated prefer-
ence models and methods;in Stated Choice Methods:Analy-
sis and Application.Edited by Louviere JJ, Hensher DA,Swait
JD. Cambridge,United Kingdom,Cambridge University Press,
2000
21.Markowitz M,Levitan B,Mohamed AF,et al:Psychiatrists’pref-
erences for benefit and risk outcomes and formulation in schizo-
phrenia treatments:a conjoint analysis study.Presented atthe
Institute on Psychiatric Services,New York,Oct 4–7,2012
22. Markowitz MA, Levitan BS,Mohamed AF,et al: Psychiatrists’
judgmentsabout antipsychotic benefitand risk outcomesand
formulation in schizophrenia treatment.Psychiatric Services 65:
1133–1139,2014
23. Dodgson JS,Spackman M,Pearman A,et al:Multi-Criteria Anal-
ysis:a Manual.London,United Kingdom,Department for Com-
munities and Local Government,2009
24. Levitan BS,Andrews EB, Gilsenan A,et al: Application ofthe
BRAT framework to case studies: observations and insights. Clinical
Pharmacology and Therapeutics 89:217–224,2011
25. BridgesJFP, Hauber AB, Marshall D, et al: Conjoint analysis
applications in health—a checklist:a reportof the ISPOR Good
Research Practices for ConjointAnalysis Task Force.Value in
Health 14:403–413,2011
26. Johnson FR, Lancsar E,Marshall D, et al: Constructing experi-
mentaldesignsfor discrete-choice experiments:report of the
ISPOR conjoint analysis experimental design good research prac-
tices task force.Value in Health 16:3–13,2013
27. Kuhfeld WF:Marketing Research Methods in SAS:Experimental
Design,Choice,Conjoint,and GraphicalTechniques.Cary,NC,
SAS Institute,2010
28. Mohamed AF,Hauber AB,Neary MP: Patientbenefit-risk pref-
erences for targeted agents in the treatment ofrenalcell carci-
noma.PharmacoEconomics 29:977–988,2011
29. Arden NK, Hauber AB,Mohamed AF,et al: How do physicians
weigh benefits and risks associated with treatments in patients
with osteoarthritis in the United Kingdom? Journalof Rheuma-
tology 39:1056–1063,2012
30. Lieberman JA,Stroup TS,McEvoy JP, et al:Effectiveness of an-
tipsychotic drugs in patients with chronic schizophrenia.New
England Journal of Medicine 353:1209–1223,2005
31.Hauber AB,Mohamed AF,Johnson FR,et al:Estimating impor-
tance weights for the IWQOL-Lite using conjoint analysis. Quality
of Life Research 19:701–709,2010
PS in Advance ps.psychiatryonline.org7
LEVITAN ET AL.
Affairs,L.L.C. Dr. Citrome is with the Departmentof Psychiatry and
BehavioralSciences,New York MedicalCollege,Valhalla.Dr. Bridges is
with the Department of Health Policy and Management, Johns Hopkins
Bloomberg Schoolof Public Health,Baltimore.
Funding for this study was obtained from Janssen Scientific Affairs, L.L.C.
The authors thank Vikram Kilambi, M.S., Angelyn Fairchild, B.A., and Gail
Zona, B.A., for their assistance in this project.The views expressed
herein do not necessarily reflect those ofJanssen Research and De-
velopment,L.L.C.,or Janssen Scientific Affairs,L.L.C.
Dr. Levitan is a stockholderin Baxter International,Inc., Johnson &
Johnson, Pharmaceutical HOLDRS Trust, and Zimmer Holdings, Inc. Dr.
Markowitz owns stock in GlaxoSmithKline,Johnson & Johnson, Pfizer,
and UCB.Ms. Mohamed holds stock in Bayer Healthcare Pharmaceut-
icals, Inc. Dr. Alphs holds stock in Johnson & Johnson. Dr. Citrome
serves as a consultant to or speaker for,or owns stock in, Actavis
(Forest),Alexza,Alkermes,AstraZeneca,Bristol-Myers Squibb,Eli Lilly,
Forum (Envivo),Genentech,Janssen, Jazz, Johnson & Johnson, Lund-
beck, Merck, Medivation, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt
Benckiser, Reviva, Shire, Sunovion, Takeda, and Teva. The other authors
report no financialrelationships with commercialinterests.
Received May 1,2014; revision received October 15,2014; accepted
December 4,2014; published online March 16,2015.
REFERENCES
1. Lehman AF, Lieberman JA, Dixon LB, et al: Practice guideline for
the treatment ofpatients with schizophrenia,2nd ed.American
Journal of Psychiatry 161(Feb suppl):1–56,2004
2. Goldner EM,Hsu L, Waraich P,et al:Prevalence and incidence
studies of schizophrenic disorders:a systematic review of the lit-
erature.Canadian Journal of Psychiatry 47:833–843,2002
3. Volavka J, Citrome L: Oral antipsychotics for the treatmentof
schizophrenia:heterogeneity in efficacy and tolerability should
drive decision-making.Expert Opinion on Pharmacotherapy 10:
1917–1928,2009
4. Citrome L: New second-generation long-acting injectable anti-
psychotics for the treatment of schizophrenia.Expert Review of
Neurotherapeutics 13:767–783,2013
5. Citrome L:Evidence-based medicine:it’s not just about the evi-
dence.International Journal of Clinical Practice 65:634–635,2011
6. Hauber AB,Arden NK,Mohamed AF,et al:A discrete-choice ex-
periment of United Kingdom patients’willingness to risk adverse
events for improved function and pain controlin osteoarthritis.
Osteoarthritis and Cartilage 21:289–297,2013
7. Bridges JF, Mohamed AF, Finnern HW, et al: Patients’ preferences
for treatment outcomes for advanced non-smallcell lung cancer:
a conjoint analysis.Lung Cancer 77:224–231,2012
8. Shumway M,Saunders T,Shern D,et al:Preferences for schizo-
phrenia treatmentoutcomes among public policy makers,con-
sumers, families, and providers. Psychiatric Services 54:1124–1128,
2003
9. Shumway M,Sentell T,Chouljian T,et al:Assessing preferences
for schizophrenia outcomes:comprehension and decision strate-
gies in three assessment methods. Mental Health Services Research
5:121–135,2003
10.Bridges JF, Kinter ET, Schmeding A, et al: Can patients diagnosed
with schizophrenia complete choice-based conjoint analysis tasks?
Patient 4:267–275,2011
11.Bridges JF, Slawik L, Schmeding A,et al:A test of concordance
between patient and psychiatrist valuations of multiple treatment
goals for schizophrenia.Health Expectations 16:164–176,2013
12.Kuhnigk O,Slawik L,Meyer J, et al:Valuation and attainment of
treatmentgoals in schizophrenia:perspectives ofpatients,rela-
tives, physicians, and payers. Journal of Psychiatric Practice 18:321–
328,2012
13.Shumway M: Preference weightsfor cost-outcome analysesof
schizophrenia treatments: comparison of four stakeholder groups.
Schizophrenia Bulletin 29:257–266,2003
14.Shumway M,Chouljian TL,Battle CL:Measuring preferences for
schizophrenia outcomes with the time tradeoff method. Journal of
Behavioral Health Services and Research 32:14–26,2005
15.Bryan S,Buxton M,Sheldon R,et al: Magnetic resonance imaging
for the investigation ofknee injuries:an investigation ofprefer-
ences.Health Economics 7:595–603,1998
16.Johnson FR, Ozdemir S,Manjunath R,et al: Factors that affect
adherence to bipolar disorder treatments:a stated-preference ap-
proach.Medical Care 45:545–552,2007
17.Clark MD, Determann D,Petrou S,et al:Discrete choice experi-
ments in health economics:a review of the literature.Pharmaco-
Economics 32:883–902,2014
18.MarshallD, Bridges JF,Hauber B,et al:Conjoint analysis appli-
cations in health—how are studies being designed and reported?
An update on current practice in the published literature between
2005 and 2008.Patient 3:249–256,2010
19.Johnson FR, Yang J-C, Mohamed AF:In defense ofimperfect
experimental designs; statistical efficiency and measurement error
in choice-format conjoint analysis. Presented at the Proceedings of
the Sawtooth Software Conference,Orlando,Fla, March 21–23,
2012
20. Louviere J, Swait J, Hensher D:Introduction to stated prefer-
ence models and methods;in Stated Choice Methods:Analy-
sis and Application.Edited by Louviere JJ, Hensher DA,Swait
JD. Cambridge,United Kingdom,Cambridge University Press,
2000
21.Markowitz M,Levitan B,Mohamed AF,et al:Psychiatrists’pref-
erences for benefit and risk outcomes and formulation in schizo-
phrenia treatments:a conjoint analysis study.Presented atthe
Institute on Psychiatric Services,New York,Oct 4–7,2012
22. Markowitz MA, Levitan BS,Mohamed AF,et al: Psychiatrists’
judgmentsabout antipsychotic benefitand risk outcomesand
formulation in schizophrenia treatment.Psychiatric Services 65:
1133–1139,2014
23. Dodgson JS,Spackman M,Pearman A,et al:Multi-Criteria Anal-
ysis:a Manual.London,United Kingdom,Department for Com-
munities and Local Government,2009
24. Levitan BS,Andrews EB, Gilsenan A,et al: Application ofthe
BRAT framework to case studies: observations and insights. Clinical
Pharmacology and Therapeutics 89:217–224,2011
25. BridgesJFP, Hauber AB, Marshall D, et al: Conjoint analysis
applications in health—a checklist:a reportof the ISPOR Good
Research Practices for ConjointAnalysis Task Force.Value in
Health 14:403–413,2011
26. Johnson FR, Lancsar E,Marshall D, et al: Constructing experi-
mentaldesignsfor discrete-choice experiments:report of the
ISPOR conjoint analysis experimental design good research prac-
tices task force.Value in Health 16:3–13,2013
27. Kuhfeld WF:Marketing Research Methods in SAS:Experimental
Design,Choice,Conjoint,and GraphicalTechniques.Cary,NC,
SAS Institute,2010
28. Mohamed AF,Hauber AB,Neary MP: Patientbenefit-risk pref-
erences for targeted agents in the treatment ofrenalcell carci-
noma.PharmacoEconomics 29:977–988,2011
29. Arden NK, Hauber AB,Mohamed AF,et al: How do physicians
weigh benefits and risks associated with treatments in patients
with osteoarthritis in the United Kingdom? Journalof Rheuma-
tology 39:1056–1063,2012
30. Lieberman JA,Stroup TS,McEvoy JP, et al:Effectiveness of an-
tipsychotic drugs in patients with chronic schizophrenia.New
England Journal of Medicine 353:1209–1223,2005
31.Hauber AB,Mohamed AF,Johnson FR,et al:Estimating impor-
tance weights for the IWQOL-Lite using conjoint analysis. Quality
of Life Research 19:701–709,2010
PS in Advance ps.psychiatryonline.org7
LEVITAN ET AL.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

32. Prelec D: A Bayesian truth serum for subjective data. Science 306:
462–466,2004
33. Billings RS,Scherer LL:The effects ofresponse mode and im-
portance on decision making strategies:judgment versus choice.
Organizational Behavior and Human Performance 41:1–19,1988
34. Nan X: Socialdistance,framing,and judgment:a construallevel
perspective.Human Communication Research 33:489–514,2007
35. Marta-Pedroso C,Freitas H,Domingos T:Testing for the survey
mode effect on contingent valuation data quality:a case study of
Web-based versus in-person interviews. Ecological Economics 62:
388–398,2007
36. Nielsen JS: Use of the Internet for willingness-to-pay survey:
a comparison of face-to-face and Web-based interviews. Resource
and Energy Economics 33:119–129,2011
37. Ettinger DS,Grunberg SM,Hauber AB,et al: Evaluation ofthe
relative importance of chemotherapeutic and antiemetic efficacy in
various oncologic settings.Supportive Care in Cancer 17:405–411,
2009
8 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
462–466,2004
33. Billings RS,Scherer LL:The effects ofresponse mode and im-
portance on decision making strategies:judgment versus choice.
Organizational Behavior and Human Performance 41:1–19,1988
34. Nan X: Socialdistance,framing,and judgment:a construallevel
perspective.Human Communication Research 33:489–514,2007
35. Marta-Pedroso C,Freitas H,Domingos T:Testing for the survey
mode effect on contingent valuation data quality:a case study of
Web-based versus in-person interviews. Ecological Economics 62:
388–398,2007
36. Nielsen JS: Use of the Internet for willingness-to-pay survey:
a comparison of face-to-face and Web-based interviews. Resource
and Energy Economics 33:119–129,2011
37. Ettinger DS,Grunberg SM,Hauber AB,et al: Evaluation ofthe
relative importance of chemotherapeutic and antiemetic efficacy in
various oncologic settings.Supportive Care in Cancer 17:405–411,
2009
8 ps.psychiatryonline.org PS in Advance
PATIENTS’PREFERENCES RELATED TO BENEFITS,RISKS,AND FORMULATIONS OF SCHIZOPHRENIA TREATMENT
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.