MED3ABS Clinical Trial Design Presentation: Huntington's Disease Study
VerifiedAdded on 2023/06/15
|10
|436
|110
Project
AI Summary
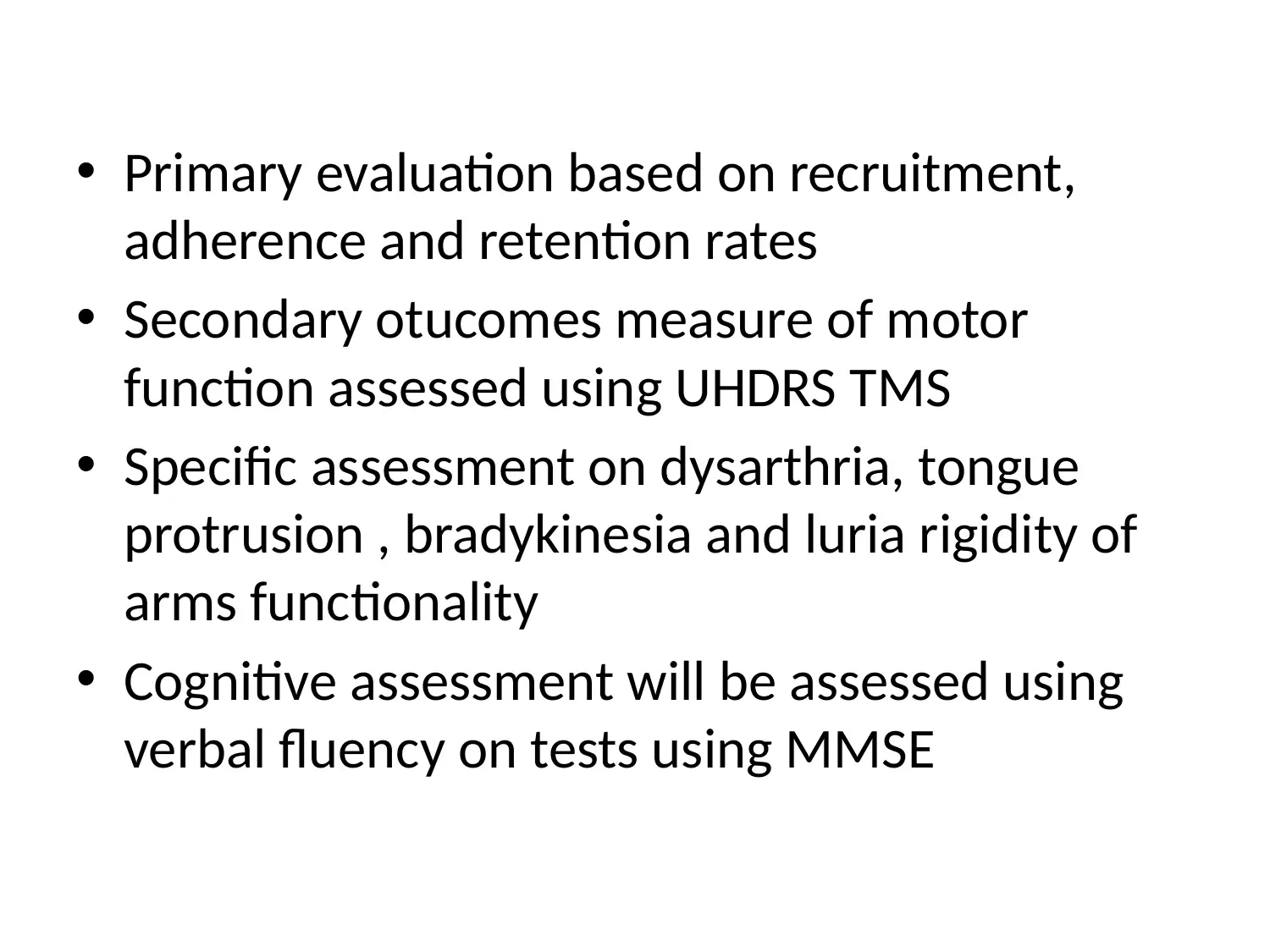
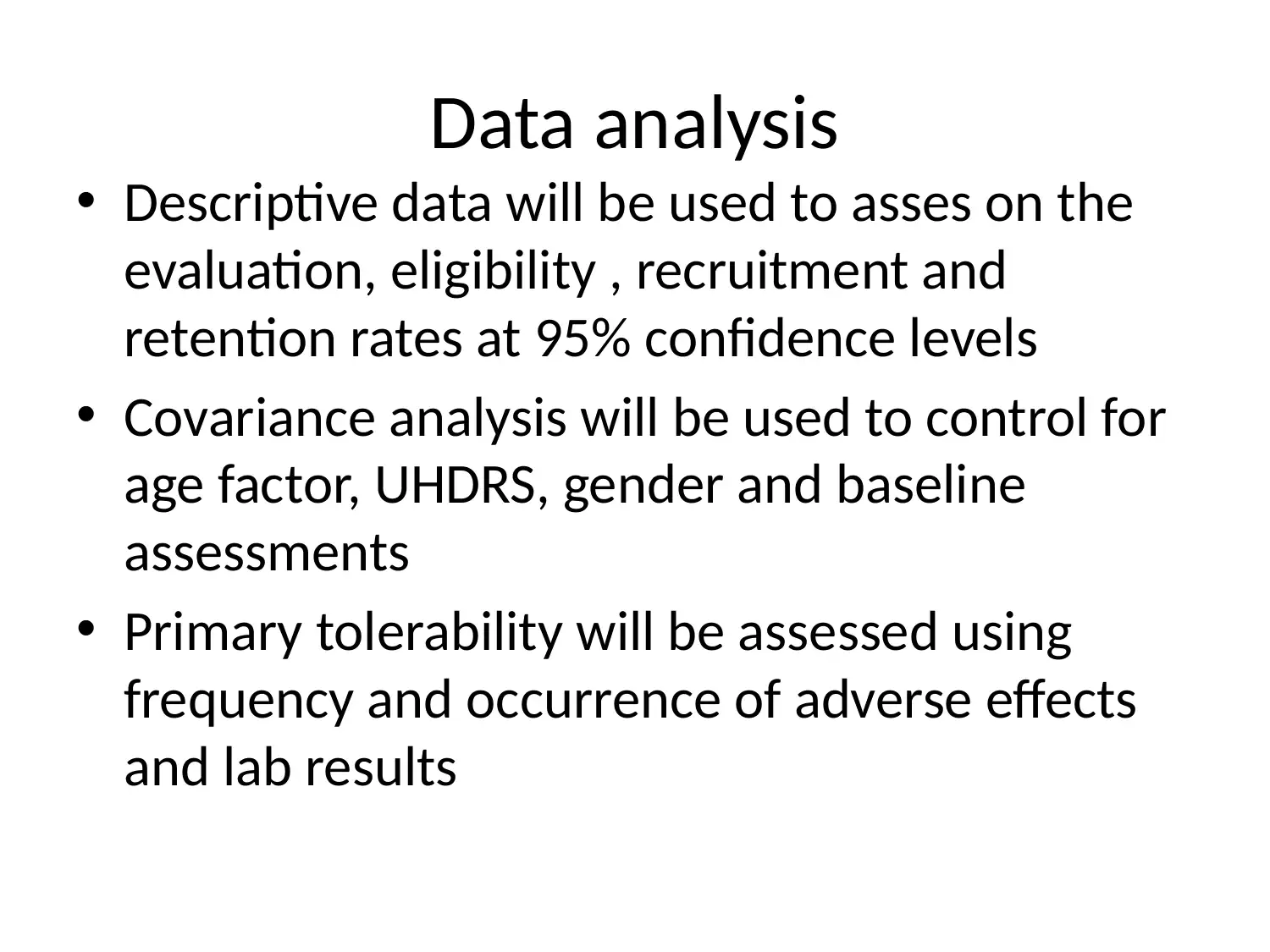
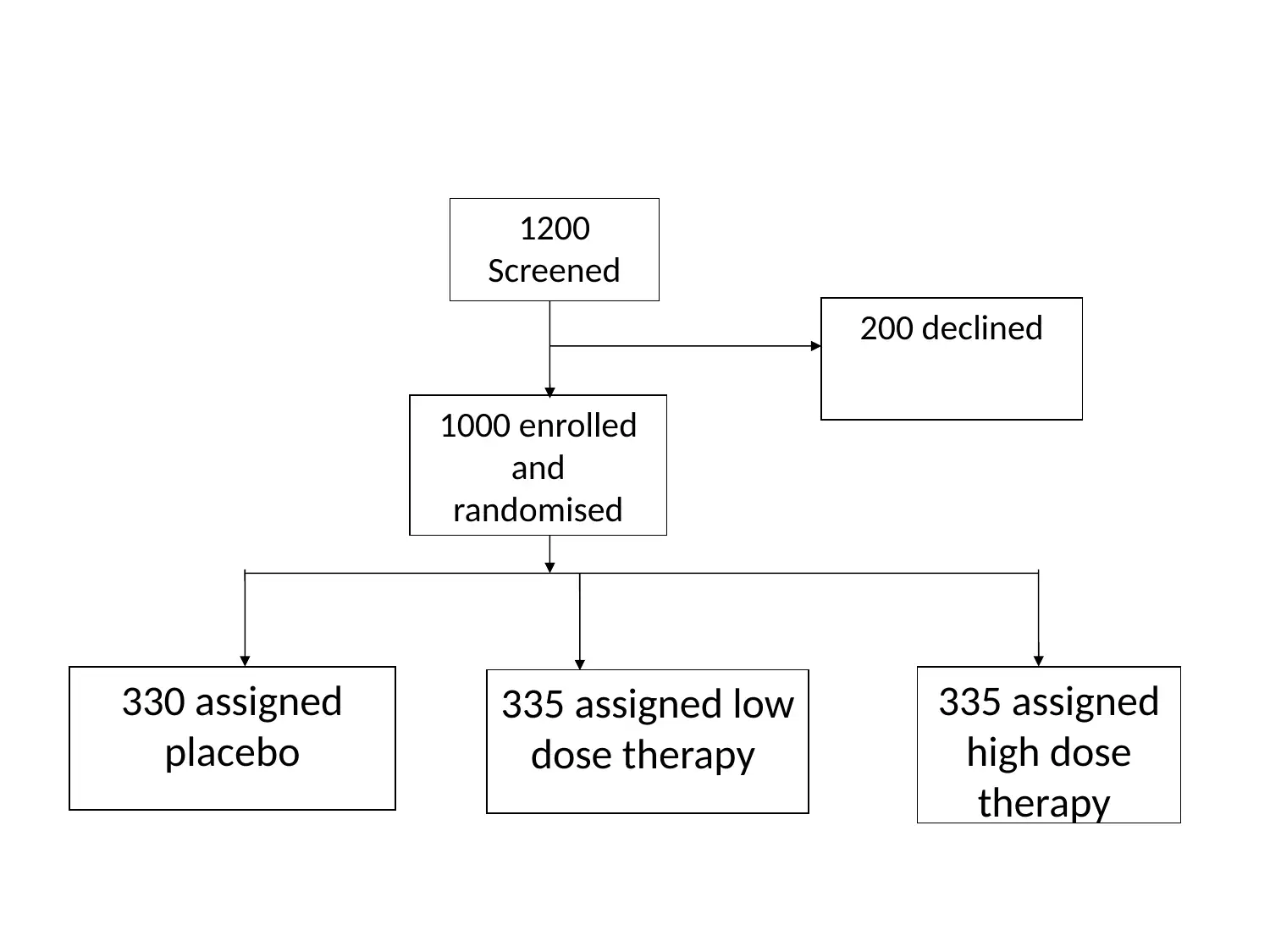
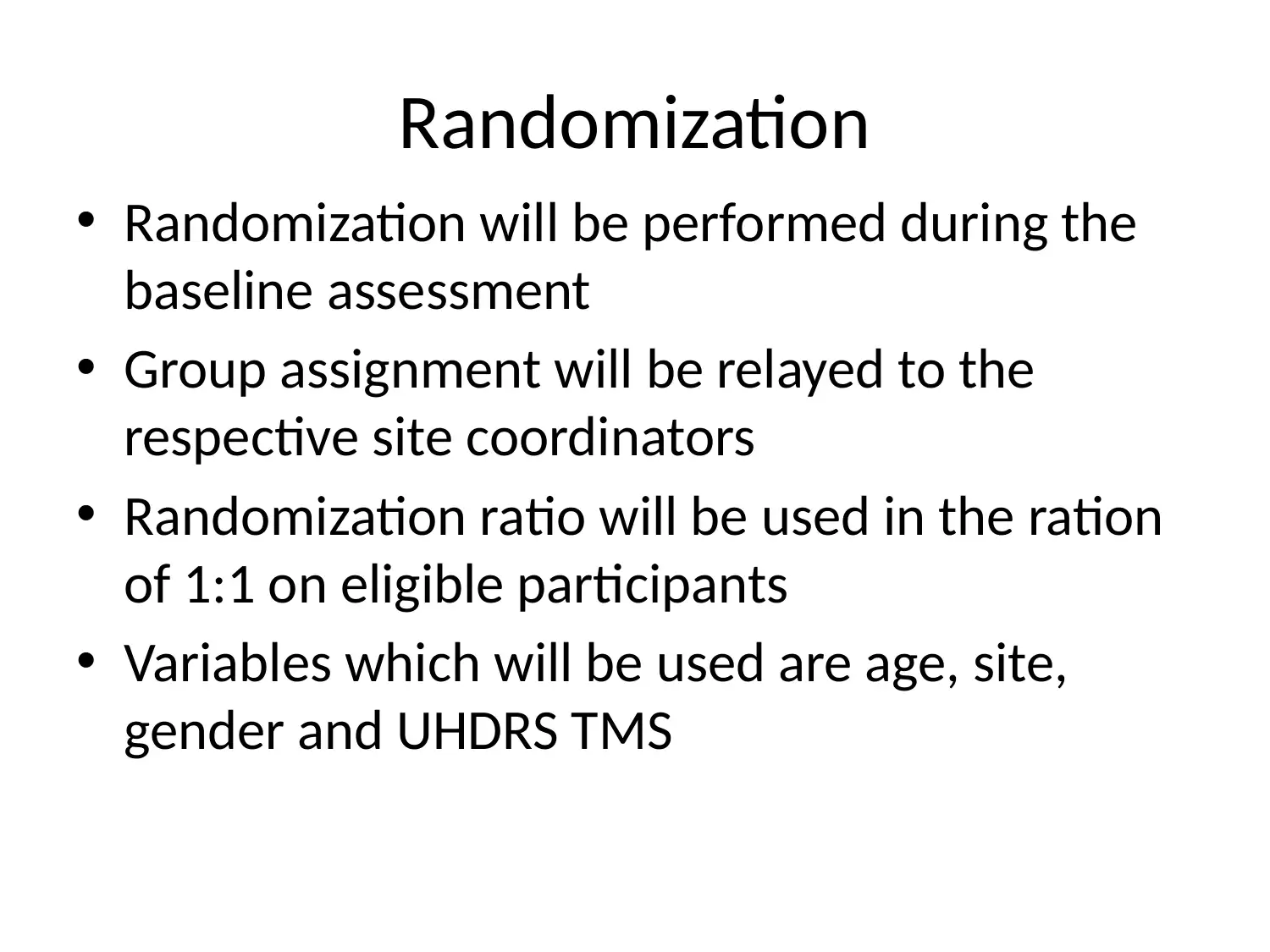
This project outlines a Phase III clinical trial design to assess the efficacy and safety of PBT2 and Beraxotene in treating Huntington's disease. The study employs a randomized, double-blinded, placebo-controlled approach, with participants divided into three groups: placebo, low-dose drug (10mg), and high-dose drug (100mg). The primary objective is to evaluate the drugs' impact on motor function and neuron survivability, measured by total functional capacity and UHDRS TMS. Secondary outcomes include assessing dysarthria, bradykinesia, and cognitive functions using MMSE. Data analysis will involve descriptive statistics and covariance analysis to control for confounding factors. The study will adhere to ethical principles for medical research, ensuring participant safety and data integrity. This document is available on Desklib, a platform offering a wide array of study tools and solved assignments for students.
1 out of 10













![[object Object]](/_next/static/media/star-bottom.7253800d.svg)