Report: Analysis of the Pharmaceutical Industry Trends and Issues
VerifiedAdded on 2023/01/11
|11
|2961
|38
Report
AI Summary
This report provides a comprehensive analysis of the pharmaceutical industry, examining its structure, trends, and challenges. It explores the industry's focus on developing prescription and over-the-counter drugs, with an emphasis on brand-name drugs and technological advancements. The report highlights the industry's global market value and growth rate, while also addressing the challenges of limited approval of new chemical entities, increased generic competition, political and regulatory impacts, and the influence of emerging markets and societal factors. It suggests strategies such as improving research and development, partnering with generic and biotech companies, and adapting to changing patient demographics and disease patterns. The report emphasizes the importance of pharmaceutical companies adapting to the evolving healthcare landscape and ensuring long-term viability by addressing stakeholder needs and societal expectations.

Pharmaceutical Industry 1
PHARMACEUTICAL INDUSTRY
By
Class (Course)
Professor (Tutor)
School (University)
City and State
The Date
PHARMACEUTICAL INDUSTRY
By
Class (Course)
Professor (Tutor)
School (University)
City and State
The Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharmaceutical Industry 2
Pharmaceutical industry
The pharmaceutical industry comprises of companies that are involved in the development of
prescription and manufacturing of over the counter products and drugs that help in preventing
illness and enhancing the quality of life in animals and human beings. In my opinion, the
pharmaceutical industry primarily focuses on companies that take an active role in developing
brand name drugs that have clear protection. The pharmaceutical industry also engages in
advancements in technology through innovative research in order to meet the population’s
complex demand in healthcare. In 2017 the pharmaceutical market globally was worth $934.8
billion. According to a recent research report on pharma market conducted by The Business
Research Company, the global pharmaceutical industry is increasing at a rate of 5.8% hence it is
expected that in 2021 it will reach $1170 billion. The rate at which the pharmaceutical industry
is expected to grow at is a bit higher compared to the other years before 2017 since it was
growing at a pace of 5.2% but compared to the other two other large healthcare segment it is
slower that is the healthcare services and medical equipment. For example, healthcare as a whole
is increasing at a rate of 7% year on year (DiMasi, Grabowski and Hansen, 2016). Having read
and analyzed the Australian pharmaceutical industry in recent years, I can conclude that it is
characterized by advancement in technology, increased competitive market and more scrutiny
from regulators, payers, and community. Hence in order for pharmaceutical companies to remain
responsive, they have opted to reassess the models of their business. But in spite of the recent
favorable developments in the pharmaceutical industry like the development of life improving
and new life-saving drugs, we still witness a system that does not operate effectively or
efficiently as per the expectations of patients, taxpayers, stakeholders and the government.
However, I still have confidence that the pharmaceutical industry can evolve in a positive path
Pharmaceutical industry
The pharmaceutical industry comprises of companies that are involved in the development of
prescription and manufacturing of over the counter products and drugs that help in preventing
illness and enhancing the quality of life in animals and human beings. In my opinion, the
pharmaceutical industry primarily focuses on companies that take an active role in developing
brand name drugs that have clear protection. The pharmaceutical industry also engages in
advancements in technology through innovative research in order to meet the population’s
complex demand in healthcare. In 2017 the pharmaceutical market globally was worth $934.8
billion. According to a recent research report on pharma market conducted by The Business
Research Company, the global pharmaceutical industry is increasing at a rate of 5.8% hence it is
expected that in 2021 it will reach $1170 billion. The rate at which the pharmaceutical industry
is expected to grow at is a bit higher compared to the other years before 2017 since it was
growing at a pace of 5.2% but compared to the other two other large healthcare segment it is
slower that is the healthcare services and medical equipment. For example, healthcare as a whole
is increasing at a rate of 7% year on year (DiMasi, Grabowski and Hansen, 2016). Having read
and analyzed the Australian pharmaceutical industry in recent years, I can conclude that it is
characterized by advancement in technology, increased competitive market and more scrutiny
from regulators, payers, and community. Hence in order for pharmaceutical companies to remain
responsive, they have opted to reassess the models of their business. But in spite of the recent
favorable developments in the pharmaceutical industry like the development of life improving
and new life-saving drugs, we still witness a system that does not operate effectively or
efficiently as per the expectations of patients, taxpayers, stakeholders and the government.
However, I still have confidence that the pharmaceutical industry can evolve in a positive path

Pharmaceutical Industry 3
by utilizing global transformations in both healthcare and technology (Lau and Dunn, 2018). I
have also realized that the big pharma and pharmaceutical industry are currently experiencing the
same conditions that the other several industries have faced in the past, and hence industries have
been enforced to try and rearrange themselves in the pursuit of adapting to the challenging
conditions they are facing. The companies that tend to modify or change their adaptability
strategies and keenly following the strategies tend to have a long term success. The issues
involved in the adaptability may include legal issue, technological issue, commercial issues, and
societal issues. There also exist a lot of strategies that can be adopted in order for pharmaceutical
industries to adjust to the demanding environment. Some adaptability strategies include
diversification, working with former competitors, merging and focusing on the emerging markets
(Ahmadiani and Nikfar, 2016).
Issues facing the pharmaceutical industry
The Approval of New Chemical Entities is limited
New Chemical Entities (NCE) involves the compounds that arise after the practice of drug
discovery. According to research carried out by the Information Management System, it
indicates that for the past ten years there has been a substantial decrease in the amount of New
Chemical Entities launched. For me, the decrease in the NCE can be attributed to the many
conditions that include increased safety standards and higher scrutiny as dictated by the Food and
Drug Administration consultants. Also, the wide-ranging collection of early-stage therapeutic
products are being looked at, but not much success is being witnessed in creating innovative
medicines in the enormous majority of the area despite processes and technology advances. I
have realized that pharmaceutical companies have to deal with the fact that there are fewer new
by utilizing global transformations in both healthcare and technology (Lau and Dunn, 2018). I
have also realized that the big pharma and pharmaceutical industry are currently experiencing the
same conditions that the other several industries have faced in the past, and hence industries have
been enforced to try and rearrange themselves in the pursuit of adapting to the challenging
conditions they are facing. The companies that tend to modify or change their adaptability
strategies and keenly following the strategies tend to have a long term success. The issues
involved in the adaptability may include legal issue, technological issue, commercial issues, and
societal issues. There also exist a lot of strategies that can be adopted in order for pharmaceutical
industries to adjust to the demanding environment. Some adaptability strategies include
diversification, working with former competitors, merging and focusing on the emerging markets
(Ahmadiani and Nikfar, 2016).
Issues facing the pharmaceutical industry
The Approval of New Chemical Entities is limited
New Chemical Entities (NCE) involves the compounds that arise after the practice of drug
discovery. According to research carried out by the Information Management System, it
indicates that for the past ten years there has been a substantial decrease in the amount of New
Chemical Entities launched. For me, the decrease in the NCE can be attributed to the many
conditions that include increased safety standards and higher scrutiny as dictated by the Food and
Drug Administration consultants. Also, the wide-ranging collection of early-stage therapeutic
products are being looked at, but not much success is being witnessed in creating innovative
medicines in the enormous majority of the area despite processes and technology advances. I
have realized that pharmaceutical companies have to deal with the fact that there are fewer new
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pharmaceutical Industry 4
products that are facing approval and hence they are deteriorating in their potential to attain
commercial benefits in their industries and provision of treatment for the patient (Schweitzer and
Lu, 2018). The pharmaceutical industries ought to find the solution for the limited approval of
new clinical entities. I feel that the first solution will begin at improving the capacity of
development and research and then followed by the business aspect. Bringing of a new medicine
into the market usually takes around 10-12 years; this involves the processes from discovery to
launch. Also, the utilization of better technology and processes and the company investing in
development and research in fixing business model can help lower the time taken from discovery
to launch (lifecycle management) of a new product. Making of better portfolio decisions can help
in enabling the companies in sharpening the focus of their investments, and when possible the
companies can look for prospects to work with other companies in order to share the business
risks involved and the cost of development and research (Schuhmacher, Gassmann and Hinder,
2016).
Increased Generic Competition
The increase in generic drugs poses a big challenge for the established pharmaceutical
companies. Big pharmaceutical companies tend to use a lot of millions of dollars and many years
from the discovery time up to the launch time. The big companies have the capability to take
advantage of their investments and hard work when their patents are effective. But as shortly as
their patents expire, the generic drugmakers take advantage of their condition, and in a period of
6 months, they are able to challenge the profit of the big pharma by producing in most instances
efficient alternatives that are of lower cost (Song and Han, 2016). According to my opinion, the
recent economic condition (downturn), lower disposable income and reforms in healthcare in
many countries, have contributed to the generic products being attractive to consumers,
products that are facing approval and hence they are deteriorating in their potential to attain
commercial benefits in their industries and provision of treatment for the patient (Schweitzer and
Lu, 2018). The pharmaceutical industries ought to find the solution for the limited approval of
new clinical entities. I feel that the first solution will begin at improving the capacity of
development and research and then followed by the business aspect. Bringing of a new medicine
into the market usually takes around 10-12 years; this involves the processes from discovery to
launch. Also, the utilization of better technology and processes and the company investing in
development and research in fixing business model can help lower the time taken from discovery
to launch (lifecycle management) of a new product. Making of better portfolio decisions can help
in enabling the companies in sharpening the focus of their investments, and when possible the
companies can look for prospects to work with other companies in order to share the business
risks involved and the cost of development and research (Schuhmacher, Gassmann and Hinder,
2016).
Increased Generic Competition
The increase in generic drugs poses a big challenge for the established pharmaceutical
companies. Big pharmaceutical companies tend to use a lot of millions of dollars and many years
from the discovery time up to the launch time. The big companies have the capability to take
advantage of their investments and hard work when their patents are effective. But as shortly as
their patents expire, the generic drugmakers take advantage of their condition, and in a period of
6 months, they are able to challenge the profit of the big pharma by producing in most instances
efficient alternatives that are of lower cost (Song and Han, 2016). According to my opinion, the
recent economic condition (downturn), lower disposable income and reforms in healthcare in
many countries, have contributed to the generic products being attractive to consumers,
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
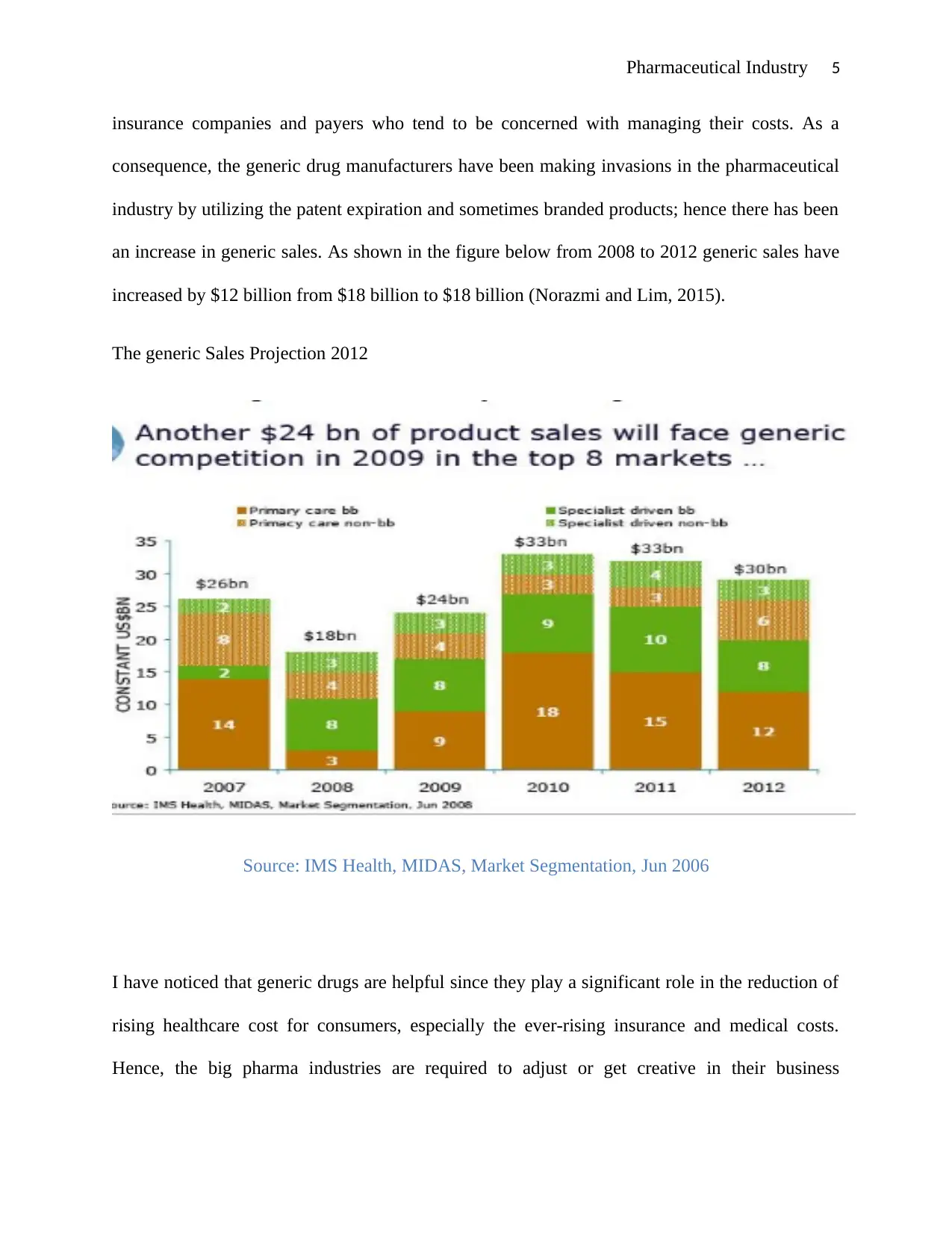
Pharmaceutical Industry 5
insurance companies and payers who tend to be concerned with managing their costs. As a
consequence, the generic drug manufacturers have been making invasions in the pharmaceutical
industry by utilizing the patent expiration and sometimes branded products; hence there has been
an increase in generic sales. As shown in the figure below from 2008 to 2012 generic sales have
increased by $12 billion from $18 billion to $18 billion (Norazmi and Lim, 2015).
The generic Sales Projection 2012
Source: IMS Health, MIDAS, Market Segmentation, Jun 2006
I have noticed that generic drugs are helpful since they play a significant role in the reduction of
rising healthcare cost for consumers, especially the ever-rising insurance and medical costs.
Hence, the big pharma industries are required to adjust or get creative in their business
insurance companies and payers who tend to be concerned with managing their costs. As a
consequence, the generic drug manufacturers have been making invasions in the pharmaceutical
industry by utilizing the patent expiration and sometimes branded products; hence there has been
an increase in generic sales. As shown in the figure below from 2008 to 2012 generic sales have
increased by $12 billion from $18 billion to $18 billion (Norazmi and Lim, 2015).
The generic Sales Projection 2012
Source: IMS Health, MIDAS, Market Segmentation, Jun 2006
I have noticed that generic drugs are helpful since they play a significant role in the reduction of
rising healthcare cost for consumers, especially the ever-rising insurance and medical costs.
Hence, the big pharma industries are required to adjust or get creative in their business

Pharmaceutical Industry 6
operations in order for them to be successful. Some of the things that the big pharma companies
can do in future in order to be successful include, partner with the generic and biotech companies
for them to easily discover additional uses and indications of their products, improve on their
product lifecycle process in order to be able to deliver additional value to patients on compounds
that exist currently. Also, they can consider emerging with their generic competence and
infrastructure so that they can gain an advantage on markets where brand drugs cost is high and
in case of expiration of the patent life (Shepherd, 2018).
Political impact and regulatory changes
The economic downturn has in several circumstances refocused and intensified the attention of
people on the pharmaceutical industry. The attention is mainly focused on improving the
regulatory process in order to meet the various requirements of the stakeholders and also for
ensuring that the benefits from the pharmaceutical industry are in line with the cost for the
services, products and insurance. I have discovered that it is a reality that the big pharmaceutical
industries are forced to restructure their costs in order to meet the patients, payers, insurance
companies and government reduced spending in the healthcare (Wu, Johan andRui, 2016). The
pharmaceutical industries should be in a position to demonstrate the value of their products to the
patients and the other concerned stakeholders especially the therapeutic products should not be
underserved. There exist organizations in countries like the Food and Drug Administration in the
United States and other similar organizations in other countries that help in ensuring the that
safety and health of the society are met (Diependaele, CockbainandSterckx, 2017). I have
realized that the Big Pharma can overcome the challenge in future by partnering with such
organizations by utilizing selected cutting edge technology that the big Pharma industries can use
to speed up their process, and it should be a win-win intention. However, this requires a greater
operations in order for them to be successful. Some of the things that the big pharma companies
can do in future in order to be successful include, partner with the generic and biotech companies
for them to easily discover additional uses and indications of their products, improve on their
product lifecycle process in order to be able to deliver additional value to patients on compounds
that exist currently. Also, they can consider emerging with their generic competence and
infrastructure so that they can gain an advantage on markets where brand drugs cost is high and
in case of expiration of the patent life (Shepherd, 2018).
Political impact and regulatory changes
The economic downturn has in several circumstances refocused and intensified the attention of
people on the pharmaceutical industry. The attention is mainly focused on improving the
regulatory process in order to meet the various requirements of the stakeholders and also for
ensuring that the benefits from the pharmaceutical industry are in line with the cost for the
services, products and insurance. I have discovered that it is a reality that the big pharmaceutical
industries are forced to restructure their costs in order to meet the patients, payers, insurance
companies and government reduced spending in the healthcare (Wu, Johan andRui, 2016). The
pharmaceutical industries should be in a position to demonstrate the value of their products to the
patients and the other concerned stakeholders especially the therapeutic products should not be
underserved. There exist organizations in countries like the Food and Drug Administration in the
United States and other similar organizations in other countries that help in ensuring the that
safety and health of the society are met (Diependaele, CockbainandSterckx, 2017). I have
realized that the Big Pharma can overcome the challenge in future by partnering with such
organizations by utilizing selected cutting edge technology that the big Pharma industries can use
to speed up their process, and it should be a win-win intention. However, this requires a greater
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pharmaceutical Industry 7
level of openness and communication than that exists currently in order to ensure that the safety
and needs of patients are of top most priority during all of the partnership and interactions. The
various groups that can have an influence on the pharmaceutical industries include Animal
Rights groups (they can resist against testing of drugs using animals) and Global warming
concerns (manufacturing plants can have some impacts on the environment). These groups and
many others tend not only to have political connections but also monetary resources hence
making it hard for big pharma industries to conduct their operations effectively in some markets
and countries (Kotabe, Jiang and Murray, 2017). The pharma companies should ensure that they
improve their relationship with the concerned groups and also understand the issues in order to
ensure that they do not experience difficulties in selling and marketing of their products after
overcoming the high obstacle of research and development and passing product safety and
effectiveness clinical trials.
The Emerging Market (Patient Demographics, Change of Diseases Patterns)
Many countries like Turkey, Russia, and India are characterized by diseases that have not been
fully understood and hence the diseases may have diverse medical needs due to differences in
climate, diet, genetics or any other factor which may tend to be distinctive in accordance to their
environment. In my opinion, it is essential that those countries have to consider investing in
investigative work and clinical trials before they embark on introducing their portfolio of current
products to the market. This necessities the need for a large scale understanding and assessment
of the various cultures, techniques of conducting business and a host of other social and
physiological factors, especially in regions where the existing population has been practicing one
type of medicine for several years (Eichler et al, 2015). In such a scenario the solution can
include the combination of new and current therapies and approaches and not merely using the
level of openness and communication than that exists currently in order to ensure that the safety
and needs of patients are of top most priority during all of the partnership and interactions. The
various groups that can have an influence on the pharmaceutical industries include Animal
Rights groups (they can resist against testing of drugs using animals) and Global warming
concerns (manufacturing plants can have some impacts on the environment). These groups and
many others tend not only to have political connections but also monetary resources hence
making it hard for big pharma industries to conduct their operations effectively in some markets
and countries (Kotabe, Jiang and Murray, 2017). The pharma companies should ensure that they
improve their relationship with the concerned groups and also understand the issues in order to
ensure that they do not experience difficulties in selling and marketing of their products after
overcoming the high obstacle of research and development and passing product safety and
effectiveness clinical trials.
The Emerging Market (Patient Demographics, Change of Diseases Patterns)
Many countries like Turkey, Russia, and India are characterized by diseases that have not been
fully understood and hence the diseases may have diverse medical needs due to differences in
climate, diet, genetics or any other factor which may tend to be distinctive in accordance to their
environment. In my opinion, it is essential that those countries have to consider investing in
investigative work and clinical trials before they embark on introducing their portfolio of current
products to the market. This necessities the need for a large scale understanding and assessment
of the various cultures, techniques of conducting business and a host of other social and
physiological factors, especially in regions where the existing population has been practicing one
type of medicine for several years (Eichler et al, 2015). In such a scenario the solution can
include the combination of new and current therapies and approaches and not merely using the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharmaceutical Industry 8
strategy of replacing the medicines that have been used for several years (Cook, 2016). The big
pharmaceutical industries have a duty to their employers, patients, stakeholders and investors to
conduct their operations in a manner that will certify their viability for a long time. It is the only
way that the companies will ensure that they continue improving and providing medicines that
the various societies and stakeholders have entrusted them with producing(David, Wolfender and
Dias, 2015) The big pharma companies should mainly diversify their operations in areas where
there are prospects to meet the needs of the people. But according to me diversifying their
operations does not mean that companies should move their operations in countries where the
cost of labor is relatively cheap hence enabling them to reduce the cost of their operations and
take advantage of supply and manufacturing chain logistics. It means that they should take into
consideration the diseases patterns. The emerging market should involve a prerequisite level of
concern of patient needs, increased latent for long term success and effective necessary business
benefits (Dodgson, 2018).
Societal factors
Given the direction, volatility and intensity of social trends, the future of the contemporary
pharmaceutical industry can only be commendably appraised by evaluation of the social context
that the pharmaceutical industry is carrying out its operations. I have realized that the social
influence is pervasive; hence pharmaceutical companies should consider the social environment
before starting an extended range planning. Through insights of need, society has significantly
portrayed a great influence in exercising public and allocation of resources over most facets of
marketing, distribution, production and innovation of drugs and using the available funds by
controlling profits and prices. Unfortunately, the future of the multinational pharmaceutical
industry indicates that the social environment has a low level of influence on the industries.
strategy of replacing the medicines that have been used for several years (Cook, 2016). The big
pharmaceutical industries have a duty to their employers, patients, stakeholders and investors to
conduct their operations in a manner that will certify their viability for a long time. It is the only
way that the companies will ensure that they continue improving and providing medicines that
the various societies and stakeholders have entrusted them with producing(David, Wolfender and
Dias, 2015) The big pharma companies should mainly diversify their operations in areas where
there are prospects to meet the needs of the people. But according to me diversifying their
operations does not mean that companies should move their operations in countries where the
cost of labor is relatively cheap hence enabling them to reduce the cost of their operations and
take advantage of supply and manufacturing chain logistics. It means that they should take into
consideration the diseases patterns. The emerging market should involve a prerequisite level of
concern of patient needs, increased latent for long term success and effective necessary business
benefits (Dodgson, 2018).
Societal factors
Given the direction, volatility and intensity of social trends, the future of the contemporary
pharmaceutical industry can only be commendably appraised by evaluation of the social context
that the pharmaceutical industry is carrying out its operations. I have realized that the social
influence is pervasive; hence pharmaceutical companies should consider the social environment
before starting an extended range planning. Through insights of need, society has significantly
portrayed a great influence in exercising public and allocation of resources over most facets of
marketing, distribution, production and innovation of drugs and using the available funds by
controlling profits and prices. Unfortunately, the future of the multinational pharmaceutical
industry indicates that the social environment has a low level of influence on the industries.

Pharmaceutical Industry 9
Operative societal forecasting techniques should be utilized in order to evaluate the effects that
the social environment has on the pharmaceutical industry. As for me, the Social environment
should be regarded as the central strategy concern and operational societal forecasting techniques
should be adopted in order for the pharmaceutical industry to be able to economically and
socially meet its commitment to add commendably to the future well-being of the society (Salton
and Jones, 2015).
Operative societal forecasting techniques should be utilized in order to evaluate the effects that
the social environment has on the pharmaceutical industry. As for me, the Social environment
should be regarded as the central strategy concern and operational societal forecasting techniques
should be adopted in order for the pharmaceutical industry to be able to economically and
socially meet its commitment to add commendably to the future well-being of the society (Salton
and Jones, 2015).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pharmaceutical Industry 10
References
Ahmadiani, S. and Nikfar, S., 2016. Challenges of access to medicine and the responsibility of
pharmaceutical companies: a legal perspective. DARU Journal of Pharmaceutical Sciences,
24(1), p.13.
Cook, A.G., 2016. Forecasting for the pharmaceutical industry: models for new product and in-
market forecasting and how to use them. Gower.
David, B., Wolfender, J.L. and Dias, D.A., 2015. The pharmaceutical industry and natural
products: historical status and new trends. Phytochemistry Reviews, 14(2), pp.299-315.
Diependaele, L., Cockbain, J. and Sterckx, S., 2017. Raising the barriers to access to medicines
in the developing world–the relentless push for data exclusivity.Developing world bioethics,
17(1), pp.11-21.
DiMasi, J.A., Grabowski, H.G. and Hansen, R.W., 2016. Innovation in the pharmaceutical
industry: new estimates of R&D costs. Journal of health economics, 47, pp.20-33.
Ding, M., Eliashberg, J. and Stremersch, S., 2016. Innovation and marketing in the
pharmaceutical industry. SPRINGER-VERLAG NEW YORK.
Dodgson, M., 2018.Technological collaboration in industry: strategy, policy and
internationalization in innovation. Routledge.
Eichler, H.G., Baird, L.G., Barker, R., Bloechl‐Daum, B., Børlum‐Kristensen, F., Brown, J.,
Chua, R., Del Signore, S., Dugan, U., Ferguson, J. and Garner, S., 2015. From adaptive licensing
to adaptive pathways: delivering a flexible life‐span approach to bring new drugs to
patients.Clinical Pharmacology & Therapeutics, 97(3), pp.234-246.
References
Ahmadiani, S. and Nikfar, S., 2016. Challenges of access to medicine and the responsibility of
pharmaceutical companies: a legal perspective. DARU Journal of Pharmaceutical Sciences,
24(1), p.13.
Cook, A.G., 2016. Forecasting for the pharmaceutical industry: models for new product and in-
market forecasting and how to use them. Gower.
David, B., Wolfender, J.L. and Dias, D.A., 2015. The pharmaceutical industry and natural
products: historical status and new trends. Phytochemistry Reviews, 14(2), pp.299-315.
Diependaele, L., Cockbain, J. and Sterckx, S., 2017. Raising the barriers to access to medicines
in the developing world–the relentless push for data exclusivity.Developing world bioethics,
17(1), pp.11-21.
DiMasi, J.A., Grabowski, H.G. and Hansen, R.W., 2016. Innovation in the pharmaceutical
industry: new estimates of R&D costs. Journal of health economics, 47, pp.20-33.
Ding, M., Eliashberg, J. and Stremersch, S., 2016. Innovation and marketing in the
pharmaceutical industry. SPRINGER-VERLAG NEW YORK.
Dodgson, M., 2018.Technological collaboration in industry: strategy, policy and
internationalization in innovation. Routledge.
Eichler, H.G., Baird, L.G., Barker, R., Bloechl‐Daum, B., Børlum‐Kristensen, F., Brown, J.,
Chua, R., Del Signore, S., Dugan, U., Ferguson, J. and Garner, S., 2015. From adaptive licensing
to adaptive pathways: delivering a flexible life‐span approach to bring new drugs to
patients.Clinical Pharmacology & Therapeutics, 97(3), pp.234-246.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pharmaceutical Industry 11
Kotabe, M., Jiang, C.X. and Murray, J.Y., 2017.Examining the complementary effect of political
networking capability with absorptive capacity on the innovative performance of emerging-
market firms.Journal of Management, 43(4), pp.1131-1156.
Lau, J.L. and Dunn, M.K., 2018. Therapeutic peptides: Historical perspectives, current
development trends, and future directions. Bioorganic & medicinal chemistry, 26(10), pp.2700-
2707.
Norazmi, M.N. and Lim, L.S., 2015. Halal pharmaceutical industry: opportunities and
challenges. Trends in pharmacological sciences, 36(8), pp.496-497.
Salton, R. and Jones, S., 2015. The corporate social responsibility reports of global
pharmaceutical firms. British Journal of Healthcare Management, 21(1), pp.21-25.
Schuhmacher, A., Gassmann, O. and Hinder, M., 2016.Changing R&D models in research-based
pharmaceutical companies.Journal of translational medicine, 14(1), p.105.
Schweitzer, S.O. and Lu, Z.J., 2018. Pharmaceutical Economics and Policy: Perspectives,
Promises, and Problems. Oxford University Press.
Shepherd, J., 2018. Consolidation and Innovation in the Pharmaceutical Industry: The Role of
Mergers and Acquisitions in the Current Innovation Ecosystem. J. Health Care L. &Pol'y, 21,
p.1.
Song, C.H. and Han, J.W., 2016. Patent cliff and strategic switch: exploring strategic design
possibilities in the pharmaceutical industry. SpringerPlus, 5(1), p.692.
Wu, W., Johan, S.A. and Rui, O.M., 2016.Institutional investors, political connections, and the
incidence of regulatory enforcement against corporate fraud.Journal of Business Ethics, 134(4),
pp.709-726.
Kotabe, M., Jiang, C.X. and Murray, J.Y., 2017.Examining the complementary effect of political
networking capability with absorptive capacity on the innovative performance of emerging-
market firms.Journal of Management, 43(4), pp.1131-1156.
Lau, J.L. and Dunn, M.K., 2018. Therapeutic peptides: Historical perspectives, current
development trends, and future directions. Bioorganic & medicinal chemistry, 26(10), pp.2700-
2707.
Norazmi, M.N. and Lim, L.S., 2015. Halal pharmaceutical industry: opportunities and
challenges. Trends in pharmacological sciences, 36(8), pp.496-497.
Salton, R. and Jones, S., 2015. The corporate social responsibility reports of global
pharmaceutical firms. British Journal of Healthcare Management, 21(1), pp.21-25.
Schuhmacher, A., Gassmann, O. and Hinder, M., 2016.Changing R&D models in research-based
pharmaceutical companies.Journal of translational medicine, 14(1), p.105.
Schweitzer, S.O. and Lu, Z.J., 2018. Pharmaceutical Economics and Policy: Perspectives,
Promises, and Problems. Oxford University Press.
Shepherd, J., 2018. Consolidation and Innovation in the Pharmaceutical Industry: The Role of
Mergers and Acquisitions in the Current Innovation Ecosystem. J. Health Care L. &Pol'y, 21,
p.1.
Song, C.H. and Han, J.W., 2016. Patent cliff and strategic switch: exploring strategic design
possibilities in the pharmaceutical industry. SpringerPlus, 5(1), p.692.
Wu, W., Johan, S.A. and Rui, O.M., 2016.Institutional investors, political connections, and the
incidence of regulatory enforcement against corporate fraud.Journal of Business Ethics, 134(4),
pp.709-726.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





