Phenolic Compounds: Sources, Toxicity, and Bioremediation Strategies
VerifiedAdded on 2023/04/06
|46
|9000
|103
Literature Review
AI Summary
This literature review provides an overview of phenolic compounds, a class of secondary plant metabolites and pollutants commonly found in industrial effluents, detailing their chemical structure, physical and chemical properties, and classification based on carbon chain, phenol units, distribution, and location in plants. It explores both natural sources, such as decomposition of organic matter and microbial action, and anthropogenic sources, including industrial, agricultural, domestic, and municipal waste, that contribute to the presence of phenolic compounds in water bodies. The review also discusses the toxicity of phenols, highlighting their adverse effects on human health, aquatic life, and the environment, and touches upon biochemical changes and health issues associated with exposure to these compounds. The document references various studies and regulatory agencies to emphasize the significance of phenolic compounds as hazardous pollutants.

Running head: PHENOL COMPOUNDS
Phenol compounds
Name of the student:
Name of the University:
Author’s note
Phenol compounds
Name of the student:
Name of the University:
Author’s note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Overview of phenolic compounds:
Phenolic compounds are class of secondary plant metabolites commonly found in
effluents coming out from oil refineries and petroleum industries and they create environment
risk by acting as one major pollutant. It also pollutes the environment by coming out through
wastewater of different industrial effluents like those producing petrochemicals, plastics,
pesticides and pharmaceuticals (Krishnaswamy Veenagayathri, 2015; Wang, Qu, 2009).
According to the European Union and the United States Environmental Protection Agency
(USEPA), the phenolic compounds are likely to have adverse effect on humans and animals.
These properties make it the most toxic primary pollutants.
According to the chemical structure of phenol compounds, it is an compound with six
membered aromatic benzene ring attached to a hydroxyl group. It is a crystalline white solid with
strong odour and most applied in liquid form. The compound is highly soluble in waters and
organic solvents. Its solubility in water is 83 G L-1 at 20 degree Celsius. The product needs
special handling techniques as it can cause burns.
Physical and chemical properties of phenol:
Phenol is mono-substituted aromatic hydrocarbon existing as white or colourless solid in
its pure state. It is commercially available as a liquid product and has sweet acrid smell detected
at 40 ppb in air. It evaporates more slowly in water. A short overview of its physical and
chemical identity is as follows:
Table 1: Physical and chemical properties of phenol. Source: (Review,
2002)
Phenolic compounds are class of secondary plant metabolites commonly found in
effluents coming out from oil refineries and petroleum industries and they create environment
risk by acting as one major pollutant. It also pollutes the environment by coming out through
wastewater of different industrial effluents like those producing petrochemicals, plastics,
pesticides and pharmaceuticals (Krishnaswamy Veenagayathri, 2015; Wang, Qu, 2009).
According to the European Union and the United States Environmental Protection Agency
(USEPA), the phenolic compounds are likely to have adverse effect on humans and animals.
These properties make it the most toxic primary pollutants.
According to the chemical structure of phenol compounds, it is an compound with six
membered aromatic benzene ring attached to a hydroxyl group. It is a crystalline white solid with
strong odour and most applied in liquid form. The compound is highly soluble in waters and
organic solvents. Its solubility in water is 83 G L-1 at 20 degree Celsius. The product needs
special handling techniques as it can cause burns.
Physical and chemical properties of phenol:
Phenol is mono-substituted aromatic hydrocarbon existing as white or colourless solid in
its pure state. It is commercially available as a liquid product and has sweet acrid smell detected
at 40 ppb in air. It evaporates more slowly in water. A short overview of its physical and
chemical identity is as follows:
Table 1: Physical and chemical properties of phenol. Source: (Review,
2002)
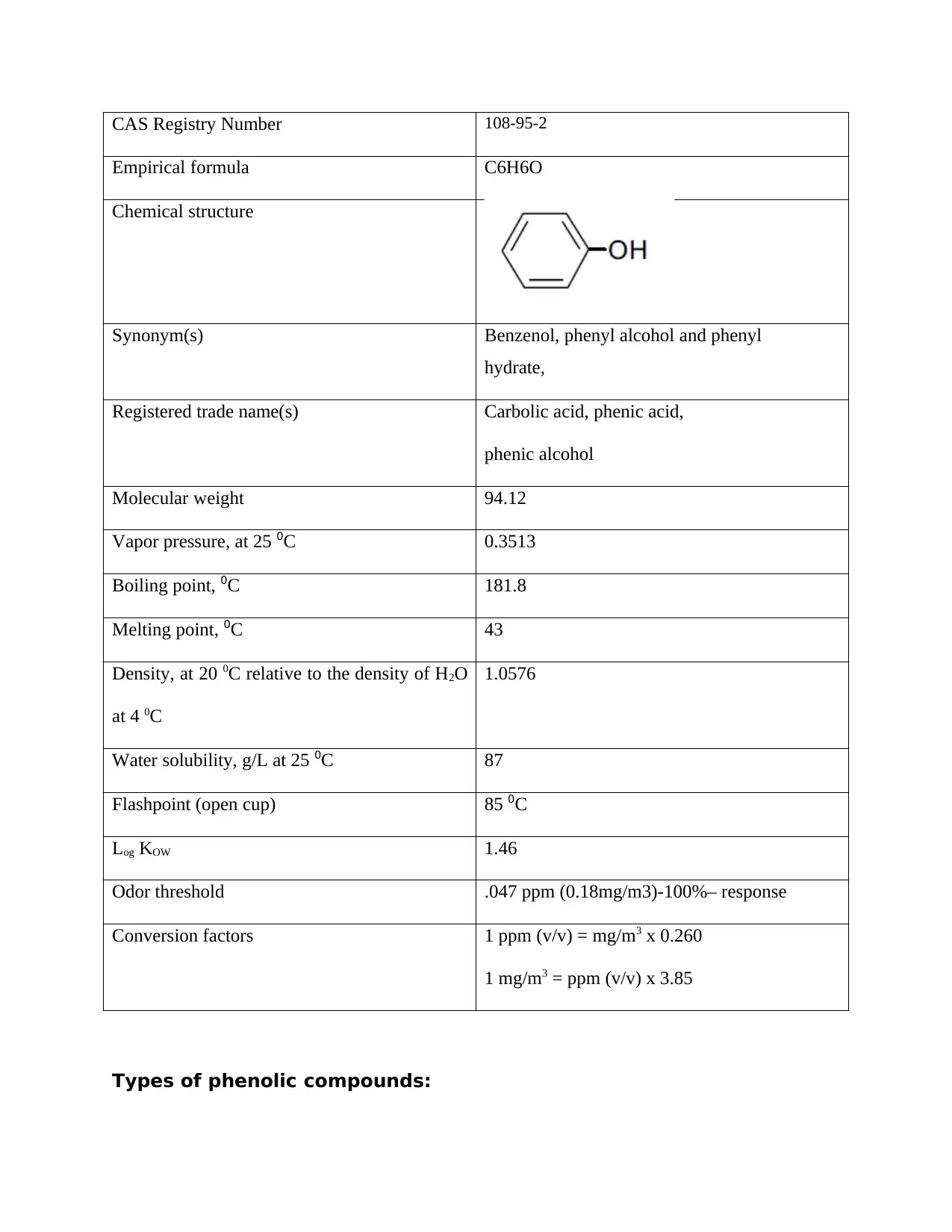
CAS Registry Number 108-95-2
Empirical formula C6H6O
Chemical structure
Synonym(s) Benzenol, phenyl alcohol and phenyl
hydrate,
Registered trade name(s) Carbolic acid, phenic acid,
phenic alcohol
Molecular weight 94.12
Vapor pressure, at 25 0C 0.3513
Boiling point, 0C 181.8
Melting point, 0C 43
Density, at 20 0C relative to the density of H2O
at 4 0C
1.0576
Water solubility, g/L at 25 0C 87
Flashpoint (open cup) 85 0C
Log KOW 1.46
Odor threshold .047 ppm (0.18mg/m3)-100%– response
Conversion factors 1 ppm (v/v) = mg/m3 x 0.260
1 mg/m3 = ppm (v/v) x 3.85
Types of phenolic compounds:
Empirical formula C6H6O
Chemical structure
Synonym(s) Benzenol, phenyl alcohol and phenyl
hydrate,
Registered trade name(s) Carbolic acid, phenic acid,
phenic alcohol
Molecular weight 94.12
Vapor pressure, at 25 0C 0.3513
Boiling point, 0C 181.8
Melting point, 0C 43
Density, at 20 0C relative to the density of H2O
at 4 0C
1.0576
Water solubility, g/L at 25 0C 87
Flashpoint (open cup) 85 0C
Log KOW 1.46
Odor threshold .047 ppm (0.18mg/m3)-100%– response
Conversion factors 1 ppm (v/v) = mg/m3 x 0.260
1 mg/m3 = ppm (v/v) x 3.85
Types of phenolic compounds:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
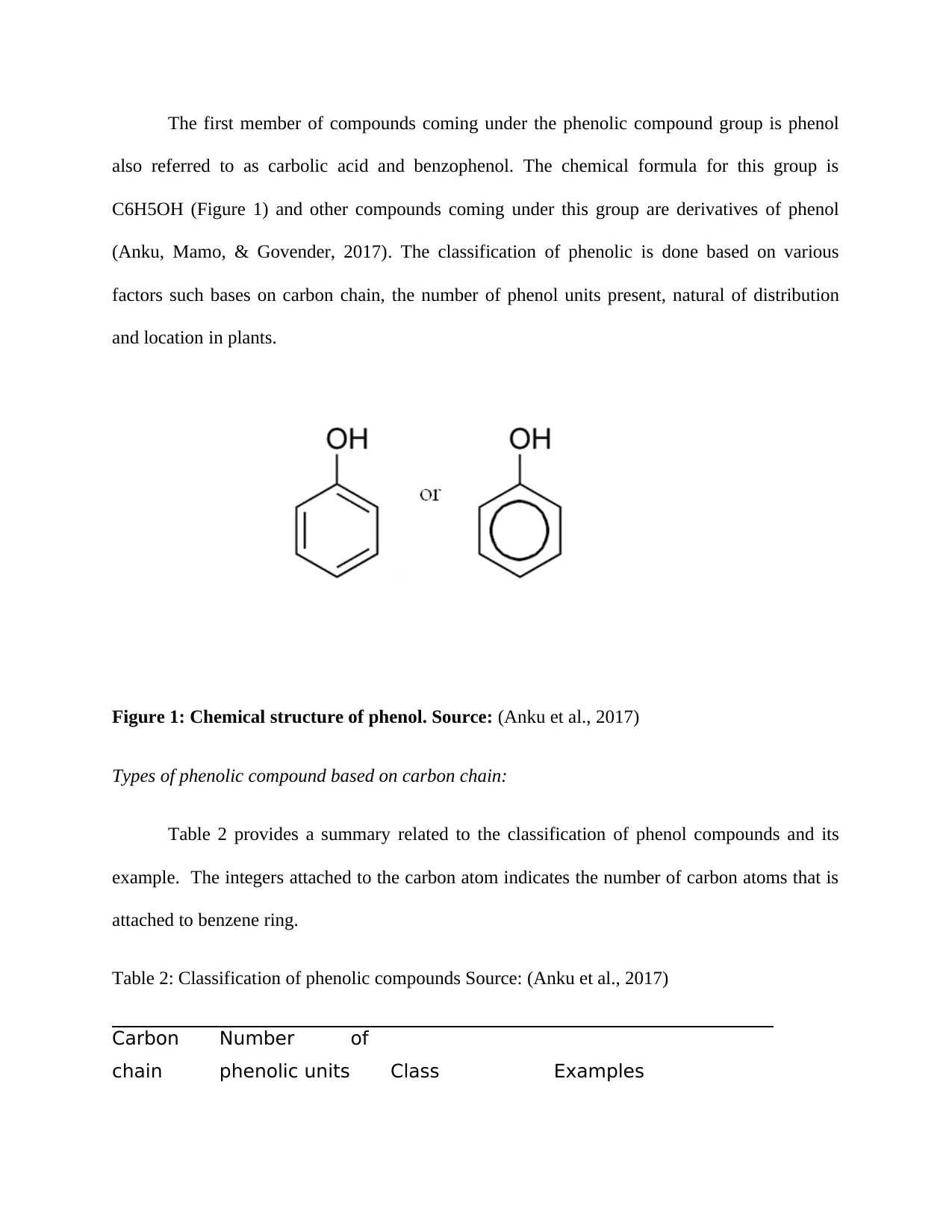
The first member of compounds coming under the phenolic compound group is phenol
also referred to as carbolic acid and benzophenol. The chemical formula for this group is
C6H5OH (Figure 1) and other compounds coming under this group are derivatives of phenol
(Anku, Mamo, & Govender, 2017). The classification of phenolic is done based on various
factors such bases on carbon chain, the number of phenol units present, natural of distribution
and location in plants.
Figure 1: Chemical structure of phenol. Source: (Anku et al., 2017)
Types of phenolic compound based on carbon chain:
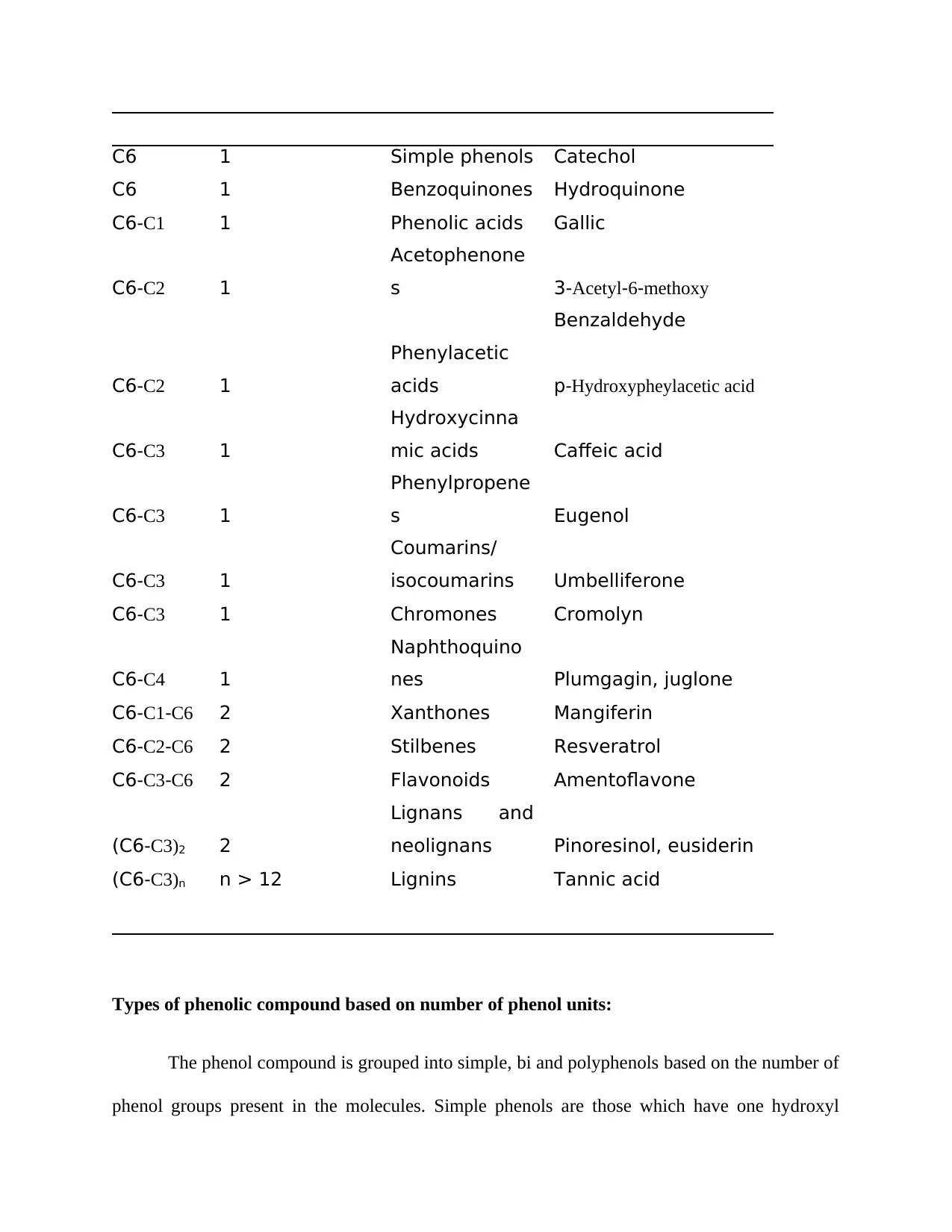
Table 2 provides a summary related to the classification of phenol compounds and its
example. The integers attached to the carbon atom indicates the number of carbon atoms that is
attached to benzene ring.
Table 2: Classification of phenolic compounds Source: (Anku et al., 2017)
Carbon
chain
Number of
phenolic units Class Examples
also referred to as carbolic acid and benzophenol. The chemical formula for this group is
C6H5OH (Figure 1) and other compounds coming under this group are derivatives of phenol
(Anku, Mamo, & Govender, 2017). The classification of phenolic is done based on various
factors such bases on carbon chain, the number of phenol units present, natural of distribution
and location in plants.
Figure 1: Chemical structure of phenol. Source: (Anku et al., 2017)
Types of phenolic compound based on carbon chain:
Table 2 provides a summary related to the classification of phenol compounds and its
example. The integers attached to the carbon atom indicates the number of carbon atoms that is
attached to benzene ring.
Table 2: Classification of phenolic compounds Source: (Anku et al., 2017)
Carbon
chain
Number of
phenolic units Class Examples
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
C6 1 Simple phenols Catechol
C6 1 Benzoquinones Hydroquinone
C6‐C1 1 Phenolic acids Gallic
C6‐C2 1
Acetophenone
s 3‐Acetyl‐6‐methoxy
Benzaldehyde
C6‐C2 1
Phenylacetic
acids p‐Hydroxypheylacetic acid
C6‐C3 1
Hydroxycinna
mic acids Caffeic acid
C6‐C3 1
Phenylpropene
s Eugenol
C6‐C3 1
Coumarins/
isocoumarins Umbelliferone
C6‐C3 1 Chromones Cromolyn
C6‐C4 1
Naphthoquino
nes Plumgagin, juglone
C6‐C1‐C6 2 Xanthones Mangiferin
C6‐C2‐C6 2 Stilbenes Resveratrol
C6‐C3‐C6 2 Flavonoids Amentoflavone
(C6‐C3)2 2
Lignans and
neolignans Pinoresinol, eusiderin
(C6‐C3)n n > 12 Lignins Tannic acid
Types of phenolic compound based on number of phenol units:
The phenol compound is grouped into simple, bi and polyphenols based on the number of
phenol groups present in the molecules. Simple phenols are those which have one hydroxyl
C6 1 Benzoquinones Hydroquinone
C6‐C1 1 Phenolic acids Gallic
C6‐C2 1
Acetophenone
s 3‐Acetyl‐6‐methoxy
Benzaldehyde
C6‐C2 1
Phenylacetic
acids p‐Hydroxypheylacetic acid
C6‐C3 1
Hydroxycinna
mic acids Caffeic acid
C6‐C3 1
Phenylpropene
s Eugenol
C6‐C3 1
Coumarins/
isocoumarins Umbelliferone
C6‐C3 1 Chromones Cromolyn
C6‐C4 1
Naphthoquino
nes Plumgagin, juglone
C6‐C1‐C6 2 Xanthones Mangiferin
C6‐C2‐C6 2 Stilbenes Resveratrol
C6‐C3‐C6 2 Flavonoids Amentoflavone
(C6‐C3)2 2
Lignans and
neolignans Pinoresinol, eusiderin
(C6‐C3)n n > 12 Lignins Tannic acid
Types of phenolic compound based on number of phenol units:
The phenol compound is grouped into simple, bi and polyphenols based on the number of
phenol groups present in the molecules. Simple phenols are those which have one hydroxyl

group attached to an aromatic ring. Examples of such compound are resorcinol, thymol and
others (Ignat, Volf & Popa, 2011). In addition, phenols with two phenolic units are known as
biphenols and those with multiple units are known as polyphenols. The polyphenols are further
classified into flavonoids, phenolic acids, tannins, lignans and stilbenes based on number of
phenol rings present and the elements attached to the ring (Archivio et al., 2007). The structure
of each type of simple phenol is illustrated in Figure 2 below:
Figure 2: Structure of simple phenol compounds Source:
Types of phenol compounds based on nature of distribution:
Phenolic compounds are classified as shortly distributed phenols and widely distributed
polymers. Shortly distributed polymers include simple phenols like pyrocatechol and resorcinol
that are less widely found in all plants. In contrast, widely distributed phenols are those that are
available in all plants such as flavonoids, coumarins and cinnamic acid. Some example polymer
class includes tannin and lignin.
Types of phenol compounds based on location in plants:
others (Ignat, Volf & Popa, 2011). In addition, phenols with two phenolic units are known as
biphenols and those with multiple units are known as polyphenols. The polyphenols are further
classified into flavonoids, phenolic acids, tannins, lignans and stilbenes based on number of
phenol rings present and the elements attached to the ring (Archivio et al., 2007). The structure
of each type of simple phenol is illustrated in Figure 2 below:
Figure 2: Structure of simple phenol compounds Source:
Types of phenol compounds based on nature of distribution:
Phenolic compounds are classified as shortly distributed phenols and widely distributed
polymers. Shortly distributed polymers include simple phenols like pyrocatechol and resorcinol
that are less widely found in all plants. In contrast, widely distributed phenols are those that are
available in all plants such as flavonoids, coumarins and cinnamic acid. Some example polymer
class includes tannin and lignin.
Types of phenol compounds based on location in plants:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Phenolic compounds that is available in soluble forms in plant cells is known as soluble
forms of phenolic compound and those bound to cell wall is known as insoluble. Soluble
compound include simple phenols, tannins and flavonoid which have low and medium molecular
weight. Example of insoluble phenolic includes those which are bound to polysaccharides in the
cell of wall of plants.
Sources of phenolic compounds in water:
Phenolic compounds exist in aquatic environment because of natural and anthropogenic
activities. Phenolic compounds derived from natural source include decayed dead plants and
animals in water. These are formed by microbial action and plants in the water. In addition,
anthropogenic source of phenolic compounds include those compounds coming from industrial,
domestic ad municipal activities. The following paragraph will provide overview of different
sources of phenols in diverse water bodies.
Source of phenolic compounds:
1. Natural source:
Decomposition or organic matter:
Phenolic compounds exist in water due to decay of deceased plants and animals in the water
bodies and by the accumulation of runoff material from land draining into water bodies. Hence,
they can be derived from plant, aquatic or terrestrial species. Some are found in hemicelluloses
part of plants under ultraviolet rays, whereas others are derived from amino acids. For example,
green and red algae have many phenolic compounds. Hydroxybenzene is synthesized via
decomposition of organic matter (Anku et al. 2017). Phenol is also produced by human body
forms of phenolic compound and those bound to cell wall is known as insoluble. Soluble
compound include simple phenols, tannins and flavonoid which have low and medium molecular
weight. Example of insoluble phenolic includes those which are bound to polysaccharides in the
cell of wall of plants.
Sources of phenolic compounds in water:
Phenolic compounds exist in aquatic environment because of natural and anthropogenic
activities. Phenolic compounds derived from natural source include decayed dead plants and
animals in water. These are formed by microbial action and plants in the water. In addition,
anthropogenic source of phenolic compounds include those compounds coming from industrial,
domestic ad municipal activities. The following paragraph will provide overview of different
sources of phenols in diverse water bodies.
Source of phenolic compounds:
1. Natural source:
Decomposition or organic matter:
Phenolic compounds exist in water due to decay of deceased plants and animals in the water
bodies and by the accumulation of runoff material from land draining into water bodies. Hence,
they can be derived from plant, aquatic or terrestrial species. Some are found in hemicelluloses
part of plants under ultraviolet rays, whereas others are derived from amino acids. For example,
green and red algae have many phenolic compounds. Hydroxybenzene is synthesized via
decomposition of organic matter (Anku et al. 2017). Phenol is also produced by human body
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

which is ultimately excreted out as phenol compounds in the form of metabolic waste. In case of
mammals, phenol is produced due to embolism of tyrosine in the digestive tract. It is found in
many fruits and vegetables and found naturally in coal tar and creosote. Benzene degradation and
natural fires also lead to production of phenolic compounds.
Action of microorganism:
Anku et al. (2017) has given evidence regarding the role of microorganism in degraded
natural substrates into phenolic compounds. For example, Debaryomyces hansenii is one
microorganism that coverts ferulic acide into various form of phenolic compounds in the
presence of glucose and nitrogen. Max, Tugores, Cortés-Diéguez, & Domínguez, 2012)
identifies that Debaryomyces hansenii produces ferulic acid, vanillin and vanillic acid and
Ghosh, Sachan, Sen, & Mitra, (2007) observed the function of Streptomyces sannanen to convert
feruic acid to vanillic acid. Phenolic compounds are also formed because by the fermentation of
plant extracts. Anku et al. (2017) studied about the use of Lentinus edodes for fermenting
cranberry pomace and Cai et al. (2012) revealed about the action of Aspergillus oryzae in
fermenting ethanolic acid and producing caffeic acid and ferulic acid.
Phenol compound synthesis by plants:
Phenol compounds are widely obtained from various plants. For example, phenolic
compounds are synthesized in chlorophyll because of the influence of stimuli like ultraviolet
radiation and microbial infection. Phenylalanine is the compound in plant that leads to phenolic
compound production. The reaction involves deamination of Phenylalanine to cinnamate through
phenylalanine ammonia lysase catalysis. This is followed by hydroxylation of cinnamate to
coumaric acid catalyzed by cinnamate-4-hydroxylase. Coumaric acid in turn leads to the
mammals, phenol is produced due to embolism of tyrosine in the digestive tract. It is found in
many fruits and vegetables and found naturally in coal tar and creosote. Benzene degradation and
natural fires also lead to production of phenolic compounds.
Action of microorganism:
Anku et al. (2017) has given evidence regarding the role of microorganism in degraded
natural substrates into phenolic compounds. For example, Debaryomyces hansenii is one
microorganism that coverts ferulic acide into various form of phenolic compounds in the
presence of glucose and nitrogen. Max, Tugores, Cortés-Diéguez, & Domínguez, 2012)
identifies that Debaryomyces hansenii produces ferulic acid, vanillin and vanillic acid and
Ghosh, Sachan, Sen, & Mitra, (2007) observed the function of Streptomyces sannanen to convert
feruic acid to vanillic acid. Phenolic compounds are also formed because by the fermentation of
plant extracts. Anku et al. (2017) studied about the use of Lentinus edodes for fermenting
cranberry pomace and Cai et al. (2012) revealed about the action of Aspergillus oryzae in
fermenting ethanolic acid and producing caffeic acid and ferulic acid.
Phenol compound synthesis by plants:
Phenol compounds are widely obtained from various plants. For example, phenolic
compounds are synthesized in chlorophyll because of the influence of stimuli like ultraviolet
radiation and microbial infection. Phenylalanine is the compound in plant that leads to phenolic
compound production. The reaction involves deamination of Phenylalanine to cinnamate through
phenylalanine ammonia lysase catalysis. This is followed by hydroxylation of cinnamate to
coumaric acid catalyzed by cinnamate-4-hydroxylase. Coumaric acid in turn leads to the

synthesis of phenolics like flavonoids and furanocoumarines (Anku et al., 2017).
The compounds are found in leaves, root and stems of plants and exudates
containing phenolic compounds. For this reason, decomposition of roots and
leave leads to the introduction of these compounds into the soil. The phenol
compounds from soil tend to wash off and run into nearby water bodies.
2. Anthropogenic source:
Industrial waste: Phenolic compounds are commonly used in daily lives particularly in different
industries involved in the production of cresols, aniline, resins and alkylphenols (Careghini,
Mastorgio, Saponaro & Sezenna, 2015). Anku et al. (2017) referred to the use of phenol
compounds in oil, gas and coal industries. Other forms of phenol used in wood and construction
industry include phenolic resins. Phenol is the raw material for many dyes, textiles and explosive
industries. Bisphenol A is another raw material that is used to manufacture non-polymer
additives, epoxy resins and polycarbonate plastics. Nylon 6 and synthetic fibres are also
produced from phenolic compounds. It is a vital constituent in pesticides and insecticides. Anku
et al. (2017) explains formation of chlorophenol during industrial activities such as distillation of
wood and paper production. Industrial effluents with phenolic compounds contribute to pollution
of the water bodies. Vehicle movement also helps in the transportation of phenolic compounds
into water bodies.
Agricultural waste: Agricultural source of water pollution includes pesticides, insecticides and
herbicides use in the field. The biodegradation of the pesticides occurs because of the detection
of phenol and chlorophenol compounds like catechols and 2‐chlorophenol, 2,4‐dichlorophenol.
Some example of pesticides include phenoxy acetic acid and 2,4‐dichlorophenoxyacetic acid.
The compounds are found in leaves, root and stems of plants and exudates
containing phenolic compounds. For this reason, decomposition of roots and
leave leads to the introduction of these compounds into the soil. The phenol
compounds from soil tend to wash off and run into nearby water bodies.
2. Anthropogenic source:
Industrial waste: Phenolic compounds are commonly used in daily lives particularly in different
industries involved in the production of cresols, aniline, resins and alkylphenols (Careghini,
Mastorgio, Saponaro & Sezenna, 2015). Anku et al. (2017) referred to the use of phenol
compounds in oil, gas and coal industries. Other forms of phenol used in wood and construction
industry include phenolic resins. Phenol is the raw material for many dyes, textiles and explosive
industries. Bisphenol A is another raw material that is used to manufacture non-polymer
additives, epoxy resins and polycarbonate plastics. Nylon 6 and synthetic fibres are also
produced from phenolic compounds. It is a vital constituent in pesticides and insecticides. Anku
et al. (2017) explains formation of chlorophenol during industrial activities such as distillation of
wood and paper production. Industrial effluents with phenolic compounds contribute to pollution
of the water bodies. Vehicle movement also helps in the transportation of phenolic compounds
into water bodies.
Agricultural waste: Agricultural source of water pollution includes pesticides, insecticides and
herbicides use in the field. The biodegradation of the pesticides occurs because of the detection
of phenol and chlorophenol compounds like catechols and 2‐chlorophenol, 2,4‐dichlorophenol.
Some example of pesticides include phenoxy acetic acid and 2,4‐dichlorophenoxyacetic acid.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Phenol compounds go into water-bodies as herbicides and pesticides as these by-products are
washed off from the field.
Domestic waste: Many household chemicals like antiseptics, slimicides, and disinfectants also
have phenol as one of the chemical constituent. Pharmaceutical products like body lotion and
mouth washes for sore treatment contain phenols, whereas household products like perfumes,
paints and varnish removers also contain phenol (Anku et al., 2017). These products are
ultimately drained into water bodies in the form household wastewater coming from washroom
and kitchens.
Municipal waste: Municipal waste treatment plants release effluents and influents and solid
landfill sites release leachates. All these are source of phenolic compounds. Leachates contain p-
cresols and they most emerge from residues of incineration wastes. Other compounds found in
leaches include 2, 4, 6-tricholorophenol,4-tertrabutylphenol and bisphenol A. In addition, other
compounds coming from municipal waste landfill sites include chlorophenols and phenols
(Kurata, Ono & Ono, 2008). Therefore, accumulation of untreated leachates, residues from
incineration site like solid fly ash flow into water bodies contribute to pollution of the aquatic
environment.
Toxicity of phenols:
Phenolic compounds are regarded as the most hazardous pollutants or contaminants
because of its toxic nature and adverse effect on human,, aquatic life and others (El-Naas, Al-
Zuhair & Alhaija, 2010). Hence, these compounds are a serious threat to environment as they
contaminate water bodies because of high water solubility and toxicity levels.
washed off from the field.
Domestic waste: Many household chemicals like antiseptics, slimicides, and disinfectants also
have phenol as one of the chemical constituent. Pharmaceutical products like body lotion and
mouth washes for sore treatment contain phenols, whereas household products like perfumes,
paints and varnish removers also contain phenol (Anku et al., 2017). These products are
ultimately drained into water bodies in the form household wastewater coming from washroom
and kitchens.
Municipal waste: Municipal waste treatment plants release effluents and influents and solid
landfill sites release leachates. All these are source of phenolic compounds. Leachates contain p-
cresols and they most emerge from residues of incineration wastes. Other compounds found in
leaches include 2, 4, 6-tricholorophenol,4-tertrabutylphenol and bisphenol A. In addition, other
compounds coming from municipal waste landfill sites include chlorophenols and phenols
(Kurata, Ono & Ono, 2008). Therefore, accumulation of untreated leachates, residues from
incineration site like solid fly ash flow into water bodies contribute to pollution of the aquatic
environment.
Toxicity of phenols:
Phenolic compounds are regarded as the most hazardous pollutants or contaminants
because of its toxic nature and adverse effect on human,, aquatic life and others (El-Naas, Al-
Zuhair & Alhaija, 2010). Hence, these compounds are a serious threat to environment as they
contaminate water bodies because of high water solubility and toxicity levels.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Other harmful impact of phenol includes several irritation to the eyes, respiratory tract
and mucous membranes because of rapid penetration through skin. For this reason, ingestion or
inhalation of phenol is associated with adverse health events and carcinogenesis too (El-Naas et
al., 2009). Examples of phenol compounds that exert toxic effect on humans include
nitrophenols, methylphenols and aminophenols. There are also compounds like alkylphenol that
exert damaging effect on endocrine glands by changing the mammary gland development in
expose animals (Banerjee, 2011). The researcher also demonstrated the action of bisphenol A in
altering pubertal timing in females. Gastrointestinal tract problem and muscle tumor difficulty
during walking is seen when drinking water is contaminated with high concentration of phenol.
Similarly, it causes great damage to the skin, heart, kidneys and liver if products containing high
level of phenol are applied by humans.
Some biochemical changes that occur due to phenol compounds include readily oxidising
to quinine radicals, a reactive compound which catalyse damage to DNAs or proteins in the
body. This process eventually disrupts the electron transportation pathway in energy transducing
membranes (Banerjee, 2011). Necrotic lesions in the stomach and mouth and throat burning are
seen due to chlorophenol poisoning. It is also associated with temperature and pulse fluctuation
and convulsions and muscle weakness (Anku et al, 2017). Another phenol compound causing
damage to the chromosomes include hydroquinone. Based different types of waste water, the
concentration of phenol vary. However, it the concentration can be as high as 4.5 g L-1 in
highly contaminated water bodies. The toxic effect of phenol is induced by
inhibiting enzyme activity. A concentration as low as 5-25 g L-1 renders lethal
effect on fish. When water contaminated with phenol is chlorinated for
disinfection, this is associated with production of high toxic polychlorinated
and mucous membranes because of rapid penetration through skin. For this reason, ingestion or
inhalation of phenol is associated with adverse health events and carcinogenesis too (El-Naas et
al., 2009). Examples of phenol compounds that exert toxic effect on humans include
nitrophenols, methylphenols and aminophenols. There are also compounds like alkylphenol that
exert damaging effect on endocrine glands by changing the mammary gland development in
expose animals (Banerjee, 2011). The researcher also demonstrated the action of bisphenol A in
altering pubertal timing in females. Gastrointestinal tract problem and muscle tumor difficulty
during walking is seen when drinking water is contaminated with high concentration of phenol.
Similarly, it causes great damage to the skin, heart, kidneys and liver if products containing high
level of phenol are applied by humans.
Some biochemical changes that occur due to phenol compounds include readily oxidising
to quinine radicals, a reactive compound which catalyse damage to DNAs or proteins in the
body. This process eventually disrupts the electron transportation pathway in energy transducing
membranes (Banerjee, 2011). Necrotic lesions in the stomach and mouth and throat burning are
seen due to chlorophenol poisoning. It is also associated with temperature and pulse fluctuation
and convulsions and muscle weakness (Anku et al, 2017). Another phenol compound causing
damage to the chromosomes include hydroquinone. Based different types of waste water, the
concentration of phenol vary. However, it the concentration can be as high as 4.5 g L-1 in
highly contaminated water bodies. The toxic effect of phenol is induced by
inhibiting enzyme activity. A concentration as low as 5-25 g L-1 renders lethal
effect on fish. When water contaminated with phenol is chlorinated for
disinfection, this is associated with production of high toxic polychlorinated

phenols. Very low concentration of phenol results in objectionable taste and poor odour to
water. The function of wastewater treatment plant seriously affected by phenol and it’s
homologous by disrupting growth of microorganism.
Because of evidence of severe toxicity of phenolic compounds, the United States
Environmental Protection Agency (USEPA) has classified phenol as a major pollutant. USEPA
has mandated achieving water purification level of less than 1 g L−1 (Keith and Telliand,
1979). The World Health Organization has also used the guideline set by USEPA to regulate
concentration of phenol in drinking water. The European Council Directive set a limit of 0.5 g
L−1 to set phenol concentration in drinking waters (Banerjee, 2011). The following table
provides a short overview of harmful effects of phenol on animals and human being:
water. The function of wastewater treatment plant seriously affected by phenol and it’s
homologous by disrupting growth of microorganism.
Because of evidence of severe toxicity of phenolic compounds, the United States
Environmental Protection Agency (USEPA) has classified phenol as a major pollutant. USEPA
has mandated achieving water purification level of less than 1 g L−1 (Keith and Telliand,
1979). The World Health Organization has also used the guideline set by USEPA to regulate
concentration of phenol in drinking water. The European Council Directive set a limit of 0.5 g
L−1 to set phenol concentration in drinking waters (Banerjee, 2011). The following table
provides a short overview of harmful effects of phenol on animals and human being:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 46
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
