The University of Sydney Physics: Electric Circuits Assignment
VerifiedAdded on 2022/10/02
|7
|1217
|160
Practical Assignment
AI Summary
This assignment explores the principles of electric circuits through an experiment and theoretical explanations. Part A focuses on investigating electrical conduction by testing various materials (copper, plastic, aluminum, and manganin) in a simple circuit, analyzing their conductivity based on light bulb brightness and current measurements. The assignment then delves into the atomic structures of these materials to explain why some are conductors (copper and aluminum) and others are insulators (plastic and manganin), using diagrams to illustrate the movement of electrons. Part B explains the heating effect of an electric current, detailing the factors involved (resistance, time, and current) and relating it to Joule's law. It describes how this effect occurs due to collisions between electrons, ions, and atoms in a conductor, and provides examples of appliances that utilize this phenomenon, supported by references.

PHYSICS-ELECTRIC CIRCUITS 1
Physics-Electric Circuits
By (Firstname Lastname)
Physics
Name of Professor
The University of Sydney
October 8, 2019
Physics-Electric Circuits
By (Firstname Lastname)
Physics
Name of Professor
The University of Sydney
October 8, 2019
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
PHYSICS-ELECTRIC CIRCUITS 2
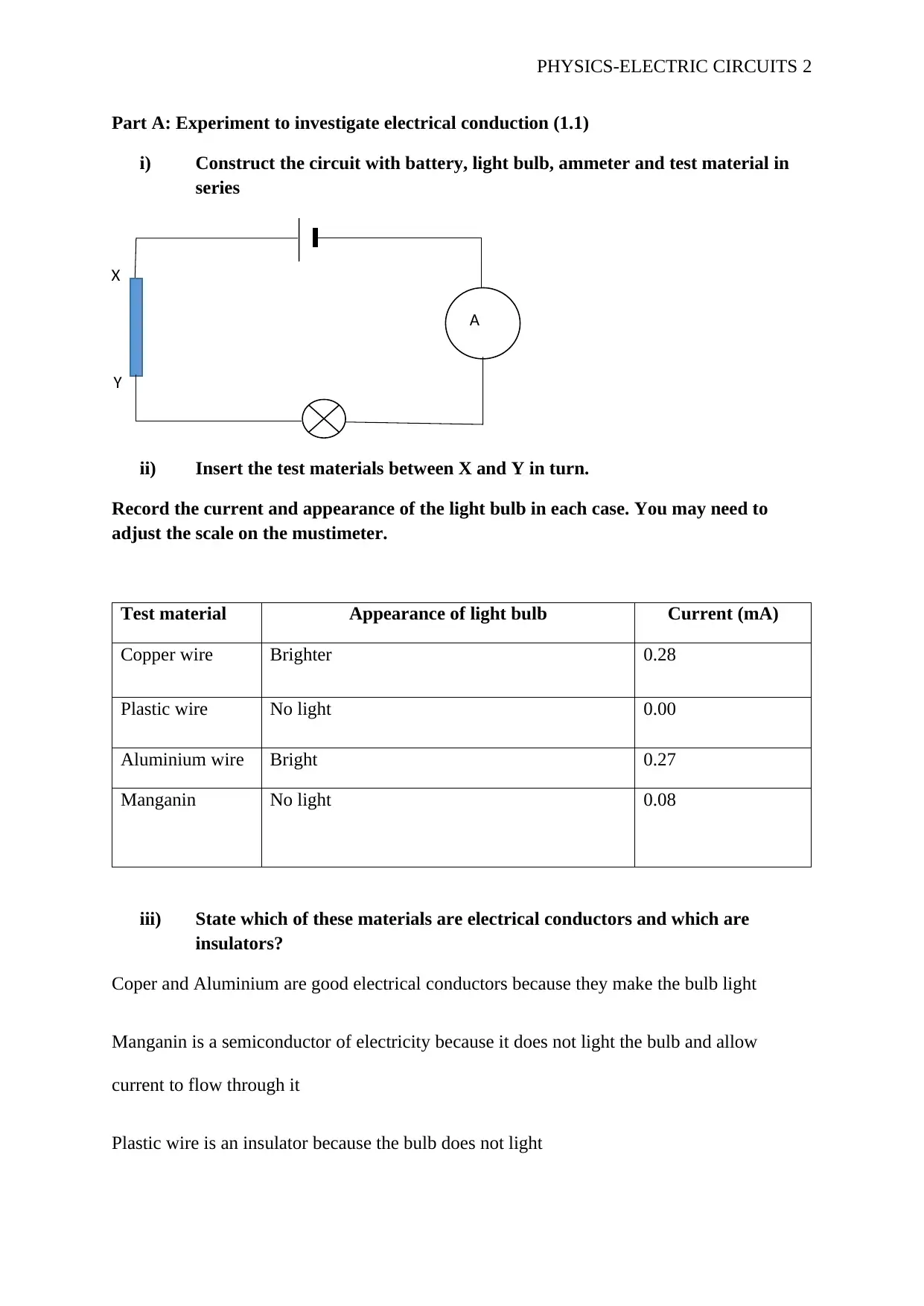
Part A: Experiment to investigate electrical conduction (1.1)
i) Construct the circuit with battery, light bulb, ammeter and test material in
series
ii) Insert the test materials between X and Y in turn.
Record the current and appearance of the light bulb in each case. You may need to
adjust the scale on the mustimeter.
Test material Appearance of light bulb Current (mA)
Copper wire Brighter 0.28
Plastic wire No light 0.00
Aluminium wire Bright 0.27
Manganin No light 0.08
iii) State which of these materials are electrical conductors and which are
insulators?
Coper and Aluminium are good electrical conductors because they make the bulb light
Manganin is a semiconductor of electricity because it does not light the bulb and allow
current to flow through it
Plastic wire is an insulator because the bulb does not light
A
Y
X
Part A: Experiment to investigate electrical conduction (1.1)
i) Construct the circuit with battery, light bulb, ammeter and test material in
series
ii) Insert the test materials between X and Y in turn.
Record the current and appearance of the light bulb in each case. You may need to
adjust the scale on the mustimeter.
Test material Appearance of light bulb Current (mA)
Copper wire Brighter 0.28
Plastic wire No light 0.00
Aluminium wire Bright 0.27
Manganin No light 0.08
iii) State which of these materials are electrical conductors and which are
insulators?
Coper and Aluminium are good electrical conductors because they make the bulb light
Manganin is a semiconductor of electricity because it does not light the bulb and allow
current to flow through it
Plastic wire is an insulator because the bulb does not light
A
Y
X

PHYSICS-ELECTRIC CIRCUITS 3
iv) Interpretation of observations
Use your knowledge of the atomic structure of these materials to explain why some
conduct electricity and some do not. You should illustrate your answer with diagrams.
Why some materials are good conductors of electricity (Copper and Aluminum)
Electrical conduction is generated by splitting and shifting electrons free from their atoms.
Atoms of certain elements very quickly let go of their outer electrons, making such elements
strong conductors. Copper is an example Some metals are protected by the same specific
ideas. The coppers atomic number is 29, indicating that it has 29 protons in the center and 29
electrons moving outside (Dyos, 2012). Copper contains two electrons throughout the shell,
eight in the shell first, eighteen in the shell fifth, and one in the shell fourth. It implies that the
first three shells increasing have almost as many electrons as they can bear, and one lone
electron is in the fourth shell. (The fourth shell can hold up to 32 electrons.) (ThoughtCo,
2019). Since this one single electron is all alone in the outer shell, it can easily distinguish
from the remainder of the atom and wander around, making copper a very strong conductor.
(Brouwer, 2011)
Atomic Structure of copper
Aluminum wire
iv) Interpretation of observations
Use your knowledge of the atomic structure of these materials to explain why some
conduct electricity and some do not. You should illustrate your answer with diagrams.
Why some materials are good conductors of electricity (Copper and Aluminum)
Electrical conduction is generated by splitting and shifting electrons free from their atoms.
Atoms of certain elements very quickly let go of their outer electrons, making such elements
strong conductors. Copper is an example Some metals are protected by the same specific
ideas. The coppers atomic number is 29, indicating that it has 29 protons in the center and 29
electrons moving outside (Dyos, 2012). Copper contains two electrons throughout the shell,
eight in the shell first, eighteen in the shell fifth, and one in the shell fourth. It implies that the
first three shells increasing have almost as many electrons as they can bear, and one lone
electron is in the fourth shell. (The fourth shell can hold up to 32 electrons.) (ThoughtCo,
2019). Since this one single electron is all alone in the outer shell, it can easily distinguish
from the remainder of the atom and wander around, making copper a very strong conductor.
(Brouwer, 2011)
Atomic Structure of copper
Aluminum wire
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
PHYSICS-ELECTRIC CIRCUITS 4
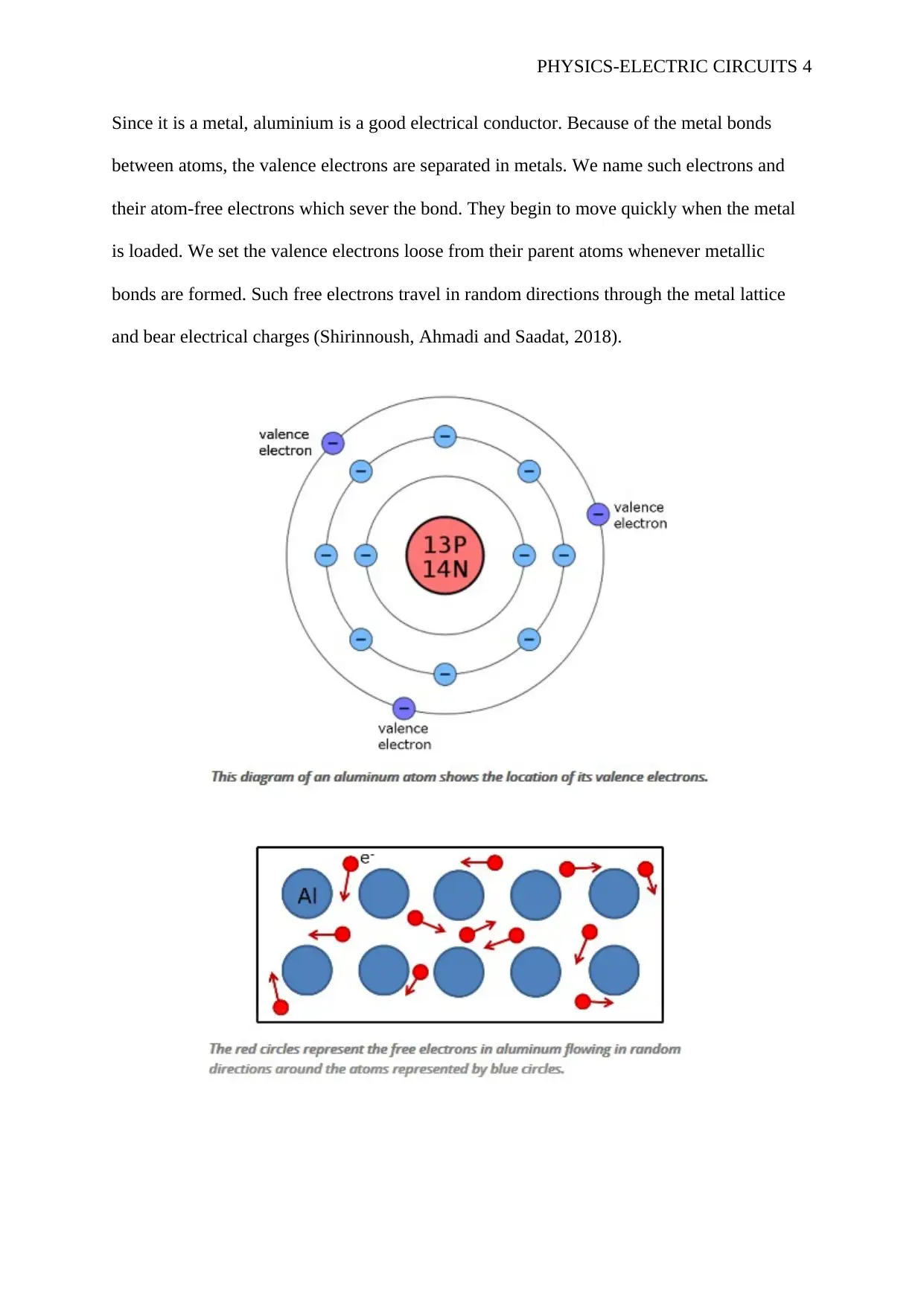
Since it is a metal, aluminium is a good electrical conductor. Because of the metal bonds
between atoms, the valence electrons are separated in metals. We name such electrons and
their atom-free electrons which sever the bond. They begin to move quickly when the metal
is loaded. We set the valence electrons loose from their parent atoms whenever metallic
bonds are formed. Such free electrons travel in random directions through the metal lattice
and bear electrical charges (Shirinnoush, Ahmadi and Saadat, 2018).
Since it is a metal, aluminium is a good electrical conductor. Because of the metal bonds
between atoms, the valence electrons are separated in metals. We name such electrons and
their atom-free electrons which sever the bond. They begin to move quickly when the metal
is loaded. We set the valence electrons loose from their parent atoms whenever metallic
bonds are formed. Such free electrons travel in random directions through the metal lattice
and bear electrical charges (Shirinnoush, Ahmadi and Saadat, 2018).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PHYSICS-ELECTRIC CIRCUITS 5
Why some materials do not conduct electricity (Plastic and Manganin)
Plastics do not have any free electrons running around so they probably wouldn't be able to
conduct heat and electricity so makes them great insulators property. The insulators are
preventing us from getting an electrical shock as they are not conducting electricity as we use
them to protect metal wires and other metallic objects. Wood, glass, rubber and water are
some other examples of good insulators. Insulators are materials which have the opposite
effect on electrons movement. We don't allow electrons to migrate from one atom to another
very easily. Insulators are materials whose electrons have been tightly bound by atoms. These
electrons aren't really free to roam and be exchanged by adjacent atoms (Setyani et al., 2017).
A semiconductor is Manganin It is a weak electricity conductor since its outer electrons are
tied up in the diamond-like framework's covalent bonds. Such atoms are bigger and have less
tight hold on their electrons. In the metallic sense of the word, they are not conductors, but
semiconductors. These are insulators at low voltages, but if the applied voltage is high
enough, these begin to conduct electricity. Electrical conductivity declines as temperature
rises in metals since atom vibrations make it harder to move electrons. For semiconductors,
this impact is offset by the larger number of electrons that are "shaken free" from bonds at
higher temperatures; as the temperature rises, semiconductors are good conductors.
Part B: Explanation of the heating effect of an electric current (1.2)
A current flowing through a conductor generates heat energy termed as the heating effect of an
electric current. these effects depend on the following factors: resistance, of the conductor, time of
the current flow, high current (profile, 2019).
According to Joule’s equation, the heating effect for a current I is given by H=I 2 Rt where R is
resistance and t is the time for heating
Why some materials do not conduct electricity (Plastic and Manganin)
Plastics do not have any free electrons running around so they probably wouldn't be able to
conduct heat and electricity so makes them great insulators property. The insulators are
preventing us from getting an electrical shock as they are not conducting electricity as we use
them to protect metal wires and other metallic objects. Wood, glass, rubber and water are
some other examples of good insulators. Insulators are materials which have the opposite
effect on electrons movement. We don't allow electrons to migrate from one atom to another
very easily. Insulators are materials whose electrons have been tightly bound by atoms. These
electrons aren't really free to roam and be exchanged by adjacent atoms (Setyani et al., 2017).
A semiconductor is Manganin It is a weak electricity conductor since its outer electrons are
tied up in the diamond-like framework's covalent bonds. Such atoms are bigger and have less
tight hold on their electrons. In the metallic sense of the word, they are not conductors, but
semiconductors. These are insulators at low voltages, but if the applied voltage is high
enough, these begin to conduct electricity. Electrical conductivity declines as temperature
rises in metals since atom vibrations make it harder to move electrons. For semiconductors,
this impact is offset by the larger number of electrons that are "shaken free" from bonds at
higher temperatures; as the temperature rises, semiconductors are good conductors.
Part B: Explanation of the heating effect of an electric current (1.2)
A current flowing through a conductor generates heat energy termed as the heating effect of an
electric current. these effects depend on the following factors: resistance, of the conductor, time of
the current flow, high current (profile, 2019).
According to Joule’s equation, the heating effect for a current I is given by H=I 2 Rt where R is
resistance and t is the time for heating

PHYSICS-ELECTRIC CIRCUITS 6
From the equation we have Joule’s law which states that amount of heat production is directly
proportional to time of flow, square of electrical current and resistance of the conductor.
The appliances that apply heating effect are electric and water heater, electric bulb, electric iron,
electric fuse. (Toppr-guides, 2019)
Heating effect is caused when a battery is connected to the ends of a conductor, and an
electric field is setup in the conductor. During this set up, the free electrons are accelerated
towards the opposite direction of the electric field, they collide with ions and atoms of the
conductor. This results into an increase in average kinetic energy of vibrations of atoms and
ions raising the temperature of the conductor
From the equation we have Joule’s law which states that amount of heat production is directly
proportional to time of flow, square of electrical current and resistance of the conductor.
The appliances that apply heating effect are electric and water heater, electric bulb, electric iron,
electric fuse. (Toppr-guides, 2019)
Heating effect is caused when a battery is connected to the ends of a conductor, and an
electric field is setup in the conductor. During this set up, the free electrons are accelerated
towards the opposite direction of the electric field, they collide with ions and atoms of the
conductor. This results into an increase in average kinetic energy of vibrations of atoms and
ions raising the temperature of the conductor
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PHYSICS-ELECTRIC CIRCUITS 7
References
Brouwer, T. (2011). Advances in electrical engineering research. New York: Nova Science
Publishers.
Dyos, G. (2012). The handbook of Electrical Resistivity. London: Institution of Engineering
and Technology.
profile, V. (2019). HEATING EFFECT OF ELECTRIC CURRENT: JOULE'S LAW. [online]
Master-scienceintro.blogspot.com. Available at:
https://master-scienceintro.blogspot.com/2011/08/heating-effect-of-electric-current.html
[Accessed 7 Oct. 2019].
Setyani, N., Suparmi, Sarwanto and Handhika, J. (2017). Students conception and perception
of simple electrical circuit. Journal of Physics: Conference Series, 909, p.012051.
Shirinnoush, S., Ahmadi, F. and Saadat, M. (2018). Transmitting energy in a simple electrical
circuit. European Journal of Physics, 39(5), p.055201.
ThoughtCo. (2019). Which Element Is the Best Conductor of Electricity?. [online] Available
at: https://www.thoughtco.com/the-most-conductive-element-606683 [Accessed 7 Oct.
2019].
Toppr-guides. (2019). Heating Effects of Electric Current and Its Applications : Concepts.
[online] Available at: https://www.toppr.com/guides/physics/electricity/heating-effects-of-
electric-current-and-its-applications/ [Accessed 7 Oct. 2019].
References
Brouwer, T. (2011). Advances in electrical engineering research. New York: Nova Science
Publishers.
Dyos, G. (2012). The handbook of Electrical Resistivity. London: Institution of Engineering
and Technology.
profile, V. (2019). HEATING EFFECT OF ELECTRIC CURRENT: JOULE'S LAW. [online]
Master-scienceintro.blogspot.com. Available at:
https://master-scienceintro.blogspot.com/2011/08/heating-effect-of-electric-current.html
[Accessed 7 Oct. 2019].
Setyani, N., Suparmi, Sarwanto and Handhika, J. (2017). Students conception and perception
of simple electrical circuit. Journal of Physics: Conference Series, 909, p.012051.
Shirinnoush, S., Ahmadi, F. and Saadat, M. (2018). Transmitting energy in a simple electrical
circuit. European Journal of Physics, 39(5), p.055201.
ThoughtCo. (2019). Which Element Is the Best Conductor of Electricity?. [online] Available
at: https://www.thoughtco.com/the-most-conductive-element-606683 [Accessed 7 Oct.
2019].
Toppr-guides. (2019). Heating Effects of Electric Current and Its Applications : Concepts.
[online] Available at: https://www.toppr.com/guides/physics/electricity/heating-effects-of-
electric-current-and-its-applications/ [Accessed 7 Oct. 2019].
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
