Is POPDC1 a Potential Therapeutic Target in Various Cancers?
VerifiedAdded on 2023/01/23
|5
|1018
|36
Report
AI Summary
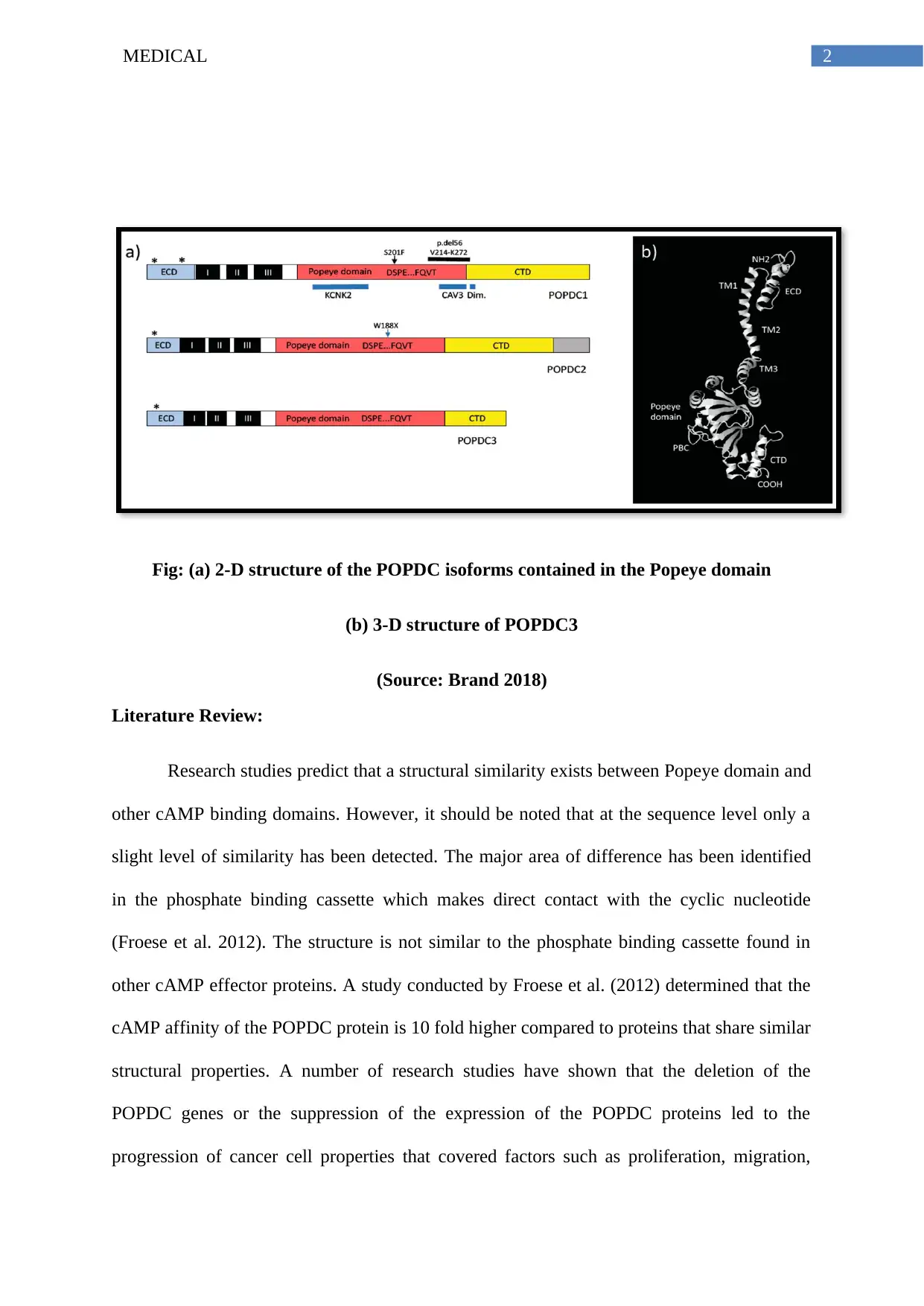
This report investigates the POPDC1 protein, a novel class of cAMP binding molecules, and its potential as a therapeutic target in various cancers. The introduction outlines the structure and function of POPDC proteins, emphasizing their role in maintaining cellular integrity. The literature review explores the relationship between POPDC proteins and cancer progression, highlighting how the deletion or suppression of POPDC genes can lead to increased proliferation, migration, and drug resistance. The report discusses the association between different POPDC proteins and specific cancers, such as breast cancer, hepatocellular carcinoma, and colorectal cancer. The conclusion suggests that POPDC1 could be a realistic drug target to prevent the loss of function and reduce the intensity of pathological consequences. The report recommends further research on the down-regulation of POPDC1 protein to provide conclusive evidence. References include key studies on POPDC proteins and their implications in cancer research.
1 out of 5








![[object Object]](/_next/static/media/star-bottom.7253800d.svg)