Experiment: Osmosis in Potato Strips with Varying Conditions
VerifiedAdded on 2021/04/24
|8
|1571
|123
Practical Assignment
AI Summary
This practical assignment details an experiment investigating osmosis using potato strips. The experiment explores the effects of varying NaCl concentrations (0g-6g) and temperatures (21°C and 42°C) on the mass change of the potato strips. The methodology involved preparing solutions, weighing potato strips, immersing them in the solutions for 20 minutes, and then re-weighing them. The results, presented in tables and graphs, demonstrate that as the NaCl concentration increases, the percentage mass change generally decreases. This is because of the reduction in water concentration. The discussion analyzes the rate of osmosis under different conditions, concluding that a saturated potato gives a faster rate of osmosis than a dehydrated potato. The conclusion summarizes the key findings, emphasizing the inverse relationship between NaCl concentration, temperature, and percentage mass change. The assignment also acknowledges potential sources of error and suggests improvements for future experiments, such as repeating the experiment multiple times for more accurate results and using potatoes of the same age. The experiment supports the understanding of osmosis as the movement of molecules from an area of less solute concentration to an area of higher solute concentration through a semi-permeable membrane.

UNIT:
NAME:
DATE:
NAME:
DATE:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

INTRODUCTION
By definition, osmosis is the net movement of solvent molecules through a semi-permeable
membrane into a region of higher solute concentration (hypertonic solution) from a region of
lower solute concentration (hypotonic solution) (Lion and Allen, 2014). Molecules tend to move
in the direction that tends to equalize the solute concentrations between the more concentrated
side and the less concentrated one(Phuntsho et al., 2012). Therefore the experiment is based on
the mass of the potato strip before and after the experiment thus a mass change if osmosis occurs.
AIMS AND HYPOTHESIS
In this experiment, the factors of osmosis under investigation are concentration of solutions and
temperature. To investigate osmosis in a potato strip, the strips would be placed in different
concentrations of NaCl, in normal water and in hot water. The expected results are that there
would be mass changes between the initial potato strips and the final strips after osmosis has
taken place. As the concentration of NaCl increases or water temperature increases, the final
mass of the strips will decrease since the strips become dehydrated.
METHODS
First, I prepared 10 solutions from distilled water and different concentrations of NaCl ranging
from 0g-6g. Of these 10 solutions, I prepared 5 solutions each with a different NaCl
concentration at a temperature of 21c˚ and the other 5 solutions, each with a different NaCl
concentration, I used hot water at temperature 42c˚. Next, I prepared 20 peeled potato strips and
weighed each of the strips and recorded their respective masses. After that, I put 2 strips of
potatoes in each solution and I left them to stay still for 20 minutes. After 20 minutes I weighed
all strips of potatoes and recorded their respective masses.
By definition, osmosis is the net movement of solvent molecules through a semi-permeable
membrane into a region of higher solute concentration (hypertonic solution) from a region of
lower solute concentration (hypotonic solution) (Lion and Allen, 2014). Molecules tend to move
in the direction that tends to equalize the solute concentrations between the more concentrated
side and the less concentrated one(Phuntsho et al., 2012). Therefore the experiment is based on
the mass of the potato strip before and after the experiment thus a mass change if osmosis occurs.
AIMS AND HYPOTHESIS
In this experiment, the factors of osmosis under investigation are concentration of solutions and
temperature. To investigate osmosis in a potato strip, the strips would be placed in different
concentrations of NaCl, in normal water and in hot water. The expected results are that there
would be mass changes between the initial potato strips and the final strips after osmosis has
taken place. As the concentration of NaCl increases or water temperature increases, the final
mass of the strips will decrease since the strips become dehydrated.
METHODS
First, I prepared 10 solutions from distilled water and different concentrations of NaCl ranging
from 0g-6g. Of these 10 solutions, I prepared 5 solutions each with a different NaCl
concentration at a temperature of 21c˚ and the other 5 solutions, each with a different NaCl
concentration, I used hot water at temperature 42c˚. Next, I prepared 20 peeled potato strips and
weighed each of the strips and recorded their respective masses. After that, I put 2 strips of
potatoes in each solution and I left them to stay still for 20 minutes. After 20 minutes I weighed
all strips of potatoes and recorded their respective masses.
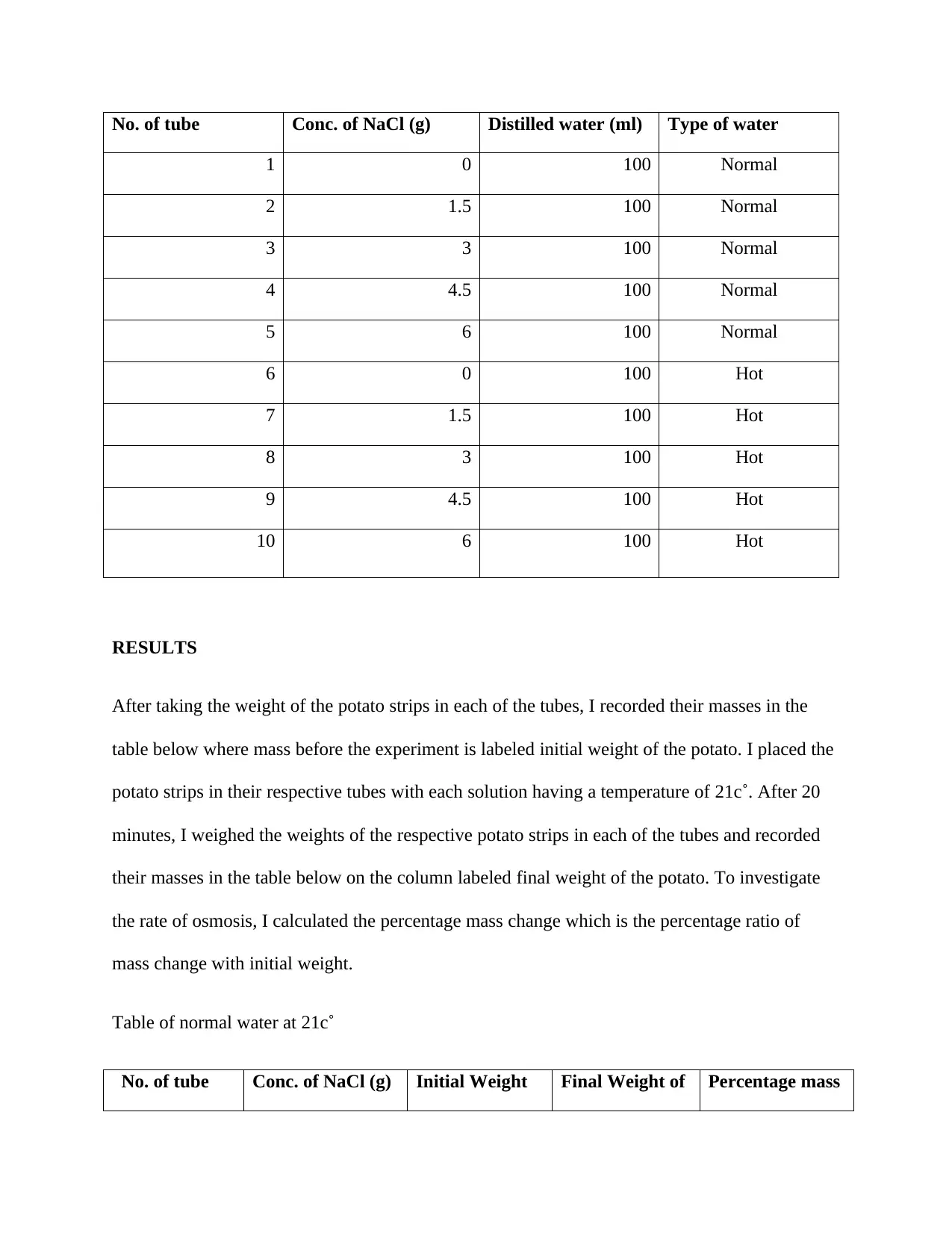
No. of tube Conc. of NaCl (g) Distilled water (ml) Type of water
1 0 100 Normal
2 1.5 100 Normal
3 3 100 Normal
4 4.5 100 Normal
5 6 100 Normal
6 0 100 Hot
7 1.5 100 Hot
8 3 100 Hot
9 4.5 100 Hot
10 6 100 Hot
RESULTS
After taking the weight of the potato strips in each of the tubes, I recorded their masses in the
table below where mass before the experiment is labeled initial weight of the potato. I placed the
potato strips in their respective tubes with each solution having a temperature of 21c˚. After 20
minutes, I weighed the weights of the respective potato strips in each of the tubes and recorded
their masses in the table below on the column labeled final weight of the potato. To investigate
the rate of osmosis, I calculated the percentage mass change which is the percentage ratio of
mass change with initial weight.
Table of normal water at 21c˚
No. of tube Conc. of NaCl (g) Initial Weight Final Weight of Percentage mass
1 0 100 Normal
2 1.5 100 Normal
3 3 100 Normal
4 4.5 100 Normal
5 6 100 Normal
6 0 100 Hot
7 1.5 100 Hot
8 3 100 Hot
9 4.5 100 Hot
10 6 100 Hot
RESULTS
After taking the weight of the potato strips in each of the tubes, I recorded their masses in the
table below where mass before the experiment is labeled initial weight of the potato. I placed the
potato strips in their respective tubes with each solution having a temperature of 21c˚. After 20
minutes, I weighed the weights of the respective potato strips in each of the tubes and recorded
their masses in the table below on the column labeled final weight of the potato. To investigate
the rate of osmosis, I calculated the percentage mass change which is the percentage ratio of
mass change with initial weight.
Table of normal water at 21c˚
No. of tube Conc. of NaCl (g) Initial Weight Final Weight of Percentage mass
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
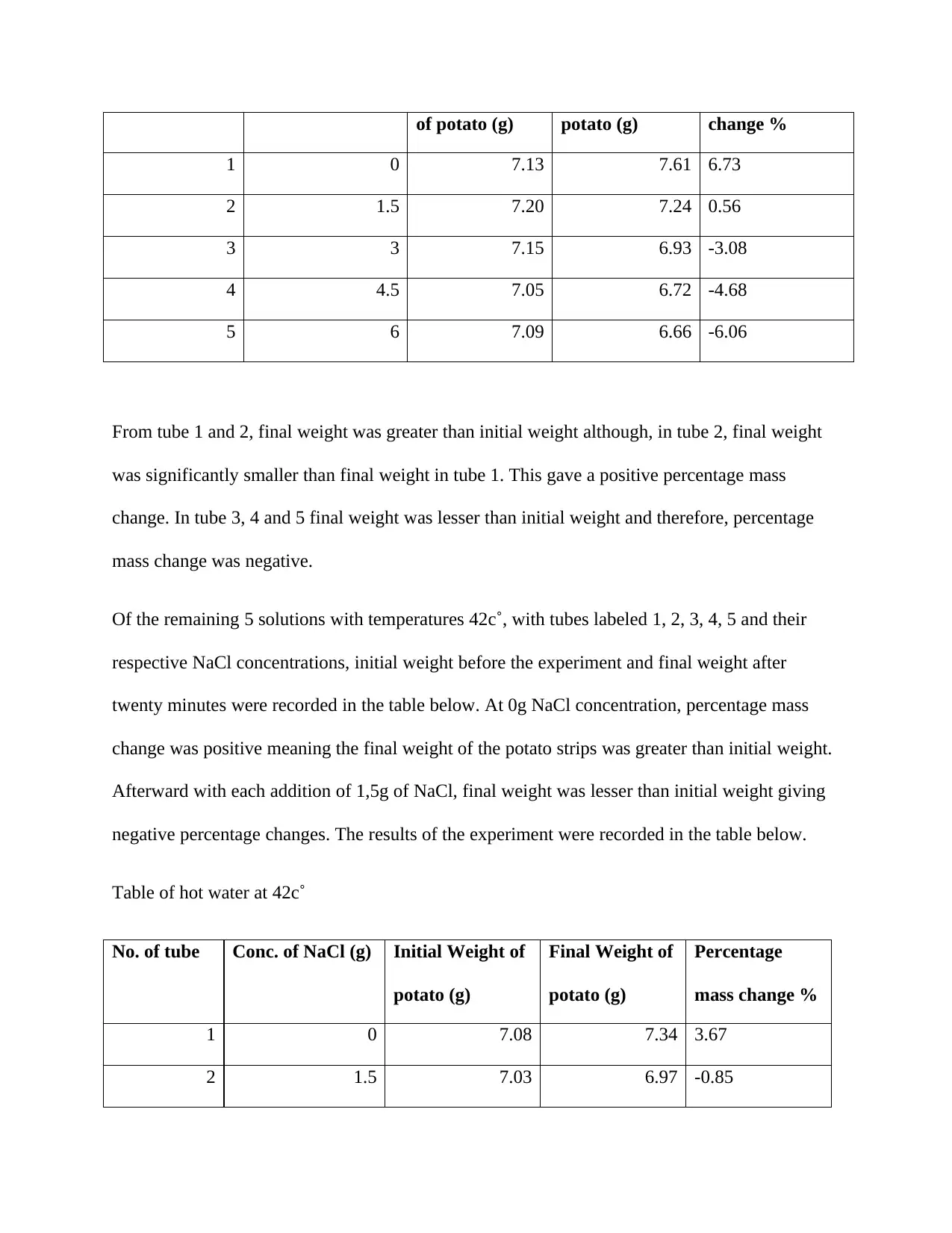
of potato (g) potato (g) change %
1 0 7.13 7.61 6.73
2 1.5 7.20 7.24 0.56
3 3 7.15 6.93 -3.08
4 4.5 7.05 6.72 -4.68
5 6 7.09 6.66 -6.06
From tube 1 and 2, final weight was greater than initial weight although, in tube 2, final weight
was significantly smaller than final weight in tube 1. This gave a positive percentage mass
change. In tube 3, 4 and 5 final weight was lesser than initial weight and therefore, percentage
mass change was negative.
Of the remaining 5 solutions with temperatures 42c˚, with tubes labeled 1, 2, 3, 4, 5 and their
respective NaCl concentrations, initial weight before the experiment and final weight after
twenty minutes were recorded in the table below. At 0g NaCl concentration, percentage mass
change was positive meaning the final weight of the potato strips was greater than initial weight.
Afterward with each addition of 1,5g of NaCl, final weight was lesser than initial weight giving
negative percentage changes. The results of the experiment were recorded in the table below.
Table of hot water at 42c˚
No. of tube Conc. of NaCl (g) Initial Weight of
potato (g)
Final Weight of
potato (g)
Percentage
mass change %
1 0 7.08 7.34 3.67
2 1.5 7.03 6.97 -0.85
1 0 7.13 7.61 6.73
2 1.5 7.20 7.24 0.56
3 3 7.15 6.93 -3.08
4 4.5 7.05 6.72 -4.68
5 6 7.09 6.66 -6.06
From tube 1 and 2, final weight was greater than initial weight although, in tube 2, final weight
was significantly smaller than final weight in tube 1. This gave a positive percentage mass
change. In tube 3, 4 and 5 final weight was lesser than initial weight and therefore, percentage
mass change was negative.
Of the remaining 5 solutions with temperatures 42c˚, with tubes labeled 1, 2, 3, 4, 5 and their
respective NaCl concentrations, initial weight before the experiment and final weight after
twenty minutes were recorded in the table below. At 0g NaCl concentration, percentage mass
change was positive meaning the final weight of the potato strips was greater than initial weight.
Afterward with each addition of 1,5g of NaCl, final weight was lesser than initial weight giving
negative percentage changes. The results of the experiment were recorded in the table below.
Table of hot water at 42c˚
No. of tube Conc. of NaCl (g) Initial Weight of
potato (g)
Final Weight of
potato (g)
Percentage
mass change %
1 0 7.08 7.34 3.67
2 1.5 7.03 6.97 -0.85
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
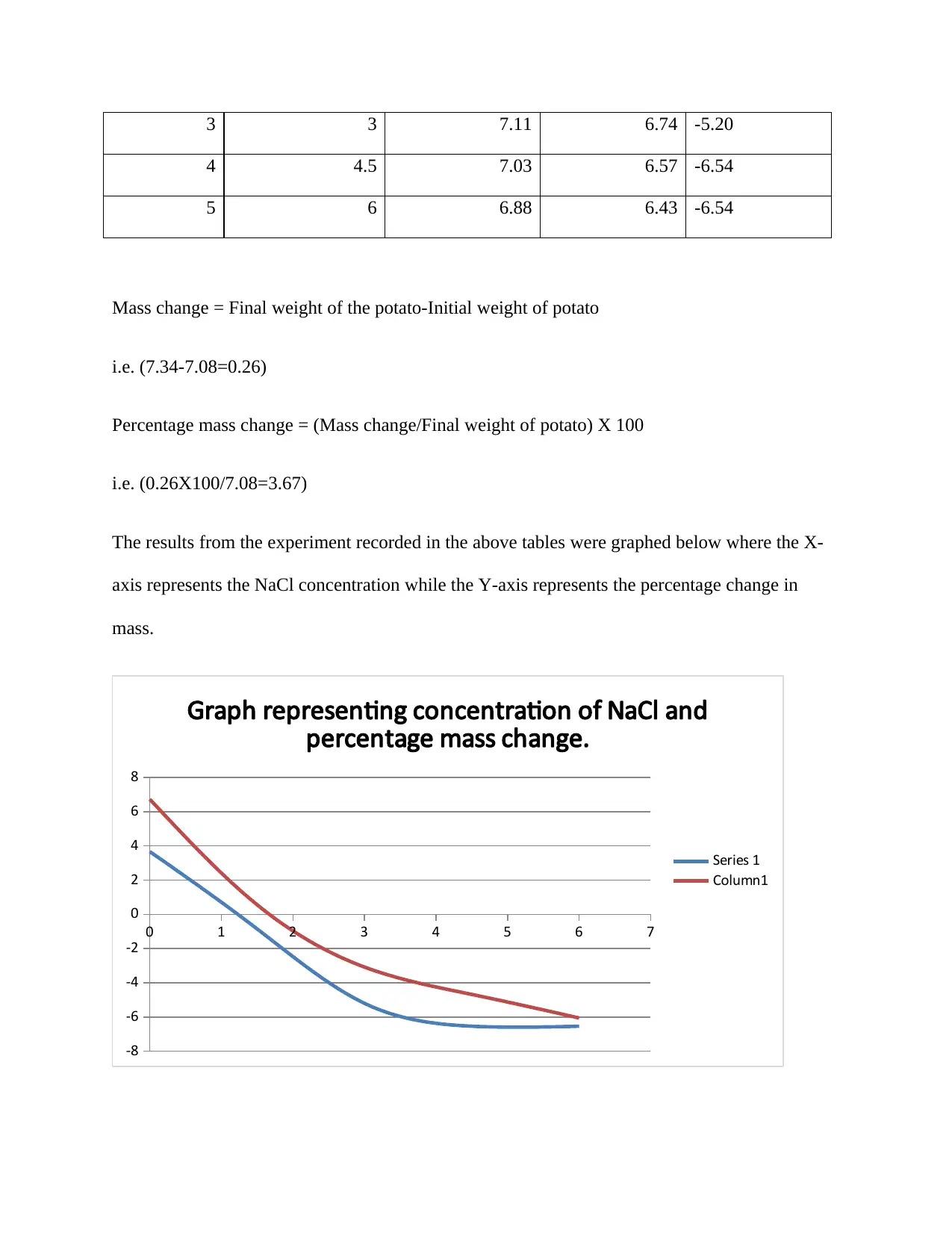
3 3 7.11 6.74 -5.20
4 4.5 7.03 6.57 -6.54
5 6 6.88 6.43 -6.54
Mass change = Final weight of the potato-Initial weight of potato
i.e. (7.34-7.08=0.26)
Percentage mass change = (Mass change/Final weight of potato) X 100
i.e. (0.26X100/7.08=3.67)
The results from the experiment recorded in the above tables were graphed below where the X-
axis represents the NaCl concentration while the Y-axis represents the percentage change in
mass.
0 1 2 3 4 5 6 7
-8
-6
-4
-2
0
2
4
6
8
Graph representing concentration of NaCl and
percentage mass change.
Series 1
Column1
4 4.5 7.03 6.57 -6.54
5 6 6.88 6.43 -6.54
Mass change = Final weight of the potato-Initial weight of potato
i.e. (7.34-7.08=0.26)
Percentage mass change = (Mass change/Final weight of potato) X 100
i.e. (0.26X100/7.08=3.67)
The results from the experiment recorded in the above tables were graphed below where the X-
axis represents the NaCl concentration while the Y-axis represents the percentage change in
mass.
0 1 2 3 4 5 6 7
-8
-6
-4
-2
0
2
4
6
8
Graph representing concentration of NaCl and
percentage mass change.
Series 1
Column1

Series 1 plots the graph for the solutions with a temperature of 42c˚ while Column 1 plots that of
solutions with a temperature of 21c˚.
DISCUSSION
Generally, as the concentration of NaCl solution increases, the percentage mass change
decreases. This is because of the fact that as the concentration of NaCl solution increases the
amount of water present in the solution or the concentration of water would be less (Kramer and
Myers, 2013). We, therefore, deduce that initially there is a gradual increase in the rate of
osmosis when the water solution has 0g NaCl. Afterward with every increase in NaCl
concentration, the rate of osmosis decreases gently as the concentration of water molecules in
and outside of the potato is about to reach the equilibrium (Alsvik and Hägg, 2013). Similarly, at
a higher temperature, increase in the concentration of NaCl solution leads to a decrease in the
percentage mass change. But in this case, the rate of osmosis decreases steeply with increase in
NaCl concentration before reaching equilibrium at NaCl concentration of 4.5g and 6g.
For NaCl concentrations of 0g-3g, on either graph, the slopes are steeper than at NaCl
concentrations of 4.5g-6g. This gives the deduction that a saturated potato gives a faster rate of
osmosis than a dehydrated potato which gives a slower rate of osmosis.
The experiment is prone to errors as the experiment is only limited to a single result without
repetition to ascertain their correctness. Repetition of a single experiment thrice say using hot
water with NaCl concentration of 1.5g would give three results and their mean would give a
more precise value as the margin of error would be smaller (Zhao and Zou, 2011). The surface
area is also a factor affecting the rate of osmosis and in this case age of the potato. The
experiment does not take into consideration the ages of the potato or potatoes used in preparing
solutions with a temperature of 21c˚.
DISCUSSION
Generally, as the concentration of NaCl solution increases, the percentage mass change
decreases. This is because of the fact that as the concentration of NaCl solution increases the
amount of water present in the solution or the concentration of water would be less (Kramer and
Myers, 2013). We, therefore, deduce that initially there is a gradual increase in the rate of
osmosis when the water solution has 0g NaCl. Afterward with every increase in NaCl
concentration, the rate of osmosis decreases gently as the concentration of water molecules in
and outside of the potato is about to reach the equilibrium (Alsvik and Hägg, 2013). Similarly, at
a higher temperature, increase in the concentration of NaCl solution leads to a decrease in the
percentage mass change. But in this case, the rate of osmosis decreases steeply with increase in
NaCl concentration before reaching equilibrium at NaCl concentration of 4.5g and 6g.
For NaCl concentrations of 0g-3g, on either graph, the slopes are steeper than at NaCl
concentrations of 4.5g-6g. This gives the deduction that a saturated potato gives a faster rate of
osmosis than a dehydrated potato which gives a slower rate of osmosis.
The experiment is prone to errors as the experiment is only limited to a single result without
repetition to ascertain their correctness. Repetition of a single experiment thrice say using hot
water with NaCl concentration of 1.5g would give three results and their mean would give a
more precise value as the margin of error would be smaller (Zhao and Zou, 2011). The surface
area is also a factor affecting the rate of osmosis and in this case age of the potato. The
experiment does not take into consideration the ages of the potato or potatoes used in preparing
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

the 20 strips under investigation. I would, therefore, use young potatoes harvested at the same
time and repeat each experiment thrice and calculate the respective means for more accurate
results.
CONCLUSION
Osmosis is the movement of molecules from an area of less solute concentration to an area of
higher solute concentration through a semi-permeable membrane. The experiment seeks to
investigate temperature and osmotic gradient as factors affecting the rate of osmosis whereby the
NaCl concentrations are altered per tube under investigation as well as temperature from 21c˚ to
42c˚. Conclusively, an increase in the concentration of NaCl in water unsaturated the potato
strips leading to a negative percentage mass change. Similarly, an increase in temperature under
the same conditions of increasing the NaCl concentration leads to a negative percentage mass
change. The difference is that the rate of osmosis would be faster at higher temperatures than at
lower temperatures since the molecules have a higher kinetic energy for collisions to happen
between water molecules at higher temperatures than at lower temperatures.
REFERENCES
Alsvik, I. L. and Hägg, M. B. (2013) ‘Pressure retarded osmosis and forward osmosis
membranes: Materials and methods’, Polymers, 5(1), pp. 303–327. doi: 10.3390/polym5010303.
Kramer, E. M. and Myers, D. R. (2013) ‘Osmosis is not driven by water dilution’, Trends in
Plant Science, pp. 195–197. doi: 10.1016/j.tplants.2012.12.001.
time and repeat each experiment thrice and calculate the respective means for more accurate
results.
CONCLUSION
Osmosis is the movement of molecules from an area of less solute concentration to an area of
higher solute concentration through a semi-permeable membrane. The experiment seeks to
investigate temperature and osmotic gradient as factors affecting the rate of osmosis whereby the
NaCl concentrations are altered per tube under investigation as well as temperature from 21c˚ to
42c˚. Conclusively, an increase in the concentration of NaCl in water unsaturated the potato
strips leading to a negative percentage mass change. Similarly, an increase in temperature under
the same conditions of increasing the NaCl concentration leads to a negative percentage mass
change. The difference is that the rate of osmosis would be faster at higher temperatures than at
lower temperatures since the molecules have a higher kinetic energy for collisions to happen
between water molecules at higher temperatures than at lower temperatures.
REFERENCES
Alsvik, I. L. and Hägg, M. B. (2013) ‘Pressure retarded osmosis and forward osmosis
membranes: Materials and methods’, Polymers, 5(1), pp. 303–327. doi: 10.3390/polym5010303.
Kramer, E. M. and Myers, D. R. (2013) ‘Osmosis is not driven by water dilution’, Trends in
Plant Science, pp. 195–197. doi: 10.1016/j.tplants.2012.12.001.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Lion, T. W. and Allen, R. J. (2014) ‘Osmosis with active solutes’, EPL, 106(3). doi:
10.1209/0295-5075/106/34003.
Phuntsho, S. et al. (2012) ‘Influence of temperature and temperature difference in the
performance of forward osmosis desalination process’, Journal of Membrane Science, 415–416,
pp. 734–744. doi: 10.1016/j.memsci.2012.05.065.
Zhao, S. and Zou, L. (2011) ‘Effects of working temperature on separation performance,
membrane scaling and cleaning in forward osmosis desalination’, Desalination, 278(1–3), pp.
157–164. doi: 10.1016/j.desal.2011.05.018.
10.1209/0295-5075/106/34003.
Phuntsho, S. et al. (2012) ‘Influence of temperature and temperature difference in the
performance of forward osmosis desalination process’, Journal of Membrane Science, 415–416,
pp. 734–744. doi: 10.1016/j.memsci.2012.05.065.
Zhao, S. and Zou, L. (2011) ‘Effects of working temperature on separation performance,
membrane scaling and cleaning in forward osmosis desalination’, Desalination, 278(1–3), pp.
157–164. doi: 10.1016/j.desal.2011.05.018.
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
