Comprehensive Evaluation of the PRNP Gene: Structure, Functionality
VerifiedAdded on 2023/06/10
|11
|1911
|481
Report
AI Summary
This report provides a detailed evaluation of the PRNP gene, which is responsible for the formation of the prion protein in humans. The report highlights the significance of the PRNP gene in synapse production, copper ion transportation, and neuroprotection. It discusses the chromosomal location of the gene (20p13) and its length, as well as the presence of exons, introns, and variants. Furthermore, the report elaborates on the structure of the prion protein, including alpha-helical strands and beta sheets, and its potential role in diseases like Creutzfeldt-Jakob disease. The analysis uses bioinformatics databases such as ENSEMBL, UniProt, and SwissProt to provide a comprehensive overview of the PRNP gene's structure, function, and potential implications in disease development, concluding that even slight conformational changes can lead to deformities.

Running head: EVALUATION OF THE PRNP GENE
EVALUATION OF THE PRNP GENE
Name of the Student:
Name of the University:
Author Note:
EVALUATION OF THE PRNP GENE
Name of the Student:
Name of the University:
Author Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1EVALUATION OF PRNP GENE
Significance of PRNP Gene:
This PRNP gene in human beings is responsible for the formation of a protein which
is known as the Prion protein. Research studies have revealed that the prion protein plays a
pivotal role in the production of synapses, which are basically the region of ‘junctions’
between neurons where communication in between the cells occur. The prion protein has also
been reported to be involved in the transportation of copper ions into the cells and ensuring
neuro-protection which basically refers to the prevention of neuronal cells from any damage
pertaining to external or internal cause (Asante et al.2015). The PRP gene is found to exist in
a variety of forms, the PRPc protein is said to be the normal expressed protein, however in
case of an abnormal expression of protein, the protein is known as PRPsc. The gene is mainly
found to be expressed intensively in the nervous system but is also found to be expressed in
other parts of the human body. It is critical here to note that the human prion protein is said to
exist in a wide range of isoforms, the normal form encompasses of the PrPc and the other
isoform PrPres is synonymous to PrPsc which is characteristically protease resistant. The
protease resistant form,that is the PrPres is primarily accountable for causing a number of
diseases in human being on the basis of the mechanism of mismatch protein folding (Brown
et al. 2015). Bovine spongiform encephalopathy, Creutzfeldt-Jakob disease, Kuru, Scrapie,
Fatal Familial Insomia and Transmissible mink encephalopathy are some of the diseases that
are found to affect human beings on account of mismatched folding of the Prion protein
(Cordeiro et al.2012). Cruetzfeldt-Jakob disease has been documented to be one of the most
fatal brain disorders according to scientific studies (Flucharty et al. 2013). The initial
symptoms incorporate impaired visibility, memory retention issues and poor coordination
between brain and muscles, however the later symptoms lead to acute dementia, complete
blindness and even complete paralysis. Almost about 90% of diagnosed cases are reported to
be dead within one year of diagnosis. This disease is caused by the manifestation of
Significance of PRNP Gene:
This PRNP gene in human beings is responsible for the formation of a protein which
is known as the Prion protein. Research studies have revealed that the prion protein plays a
pivotal role in the production of synapses, which are basically the region of ‘junctions’
between neurons where communication in between the cells occur. The prion protein has also
been reported to be involved in the transportation of copper ions into the cells and ensuring
neuro-protection which basically refers to the prevention of neuronal cells from any damage
pertaining to external or internal cause (Asante et al.2015). The PRP gene is found to exist in
a variety of forms, the PRPc protein is said to be the normal expressed protein, however in
case of an abnormal expression of protein, the protein is known as PRPsc. The gene is mainly
found to be expressed intensively in the nervous system but is also found to be expressed in
other parts of the human body. It is critical here to note that the human prion protein is said to
exist in a wide range of isoforms, the normal form encompasses of the PrPc and the other
isoform PrPres is synonymous to PrPsc which is characteristically protease resistant. The
protease resistant form,that is the PrPres is primarily accountable for causing a number of
diseases in human being on the basis of the mechanism of mismatch protein folding (Brown
et al. 2015). Bovine spongiform encephalopathy, Creutzfeldt-Jakob disease, Kuru, Scrapie,
Fatal Familial Insomia and Transmissible mink encephalopathy are some of the diseases that
are found to affect human beings on account of mismatched folding of the Prion protein
(Cordeiro et al.2012). Cruetzfeldt-Jakob disease has been documented to be one of the most
fatal brain disorders according to scientific studies (Flucharty et al. 2013). The initial
symptoms incorporate impaired visibility, memory retention issues and poor coordination
between brain and muscles, however the later symptoms lead to acute dementia, complete
blindness and even complete paralysis. Almost about 90% of diagnosed cases are reported to
be dead within one year of diagnosis. This disease is caused by the manifestation of
2EVALUATION OF PRNP GENE
infectious PRNP proteins that have the ability to distort normal conformation of the Prion
protein and mutate them into mismatched forms. Also, scientific evidences suggest that, more
than 70% of the cases occur spontaneously in the body under natural circumstances, about
(7.5-8)% cases occur due to inheritance of autosomal dominant genes with infected PRNP
gene (Gill et al.2013). The exact physiology of the PRNP gene is not known yet, however
scientific research papers reveal the direct link of PRNP gene to cause several types of
cancer.
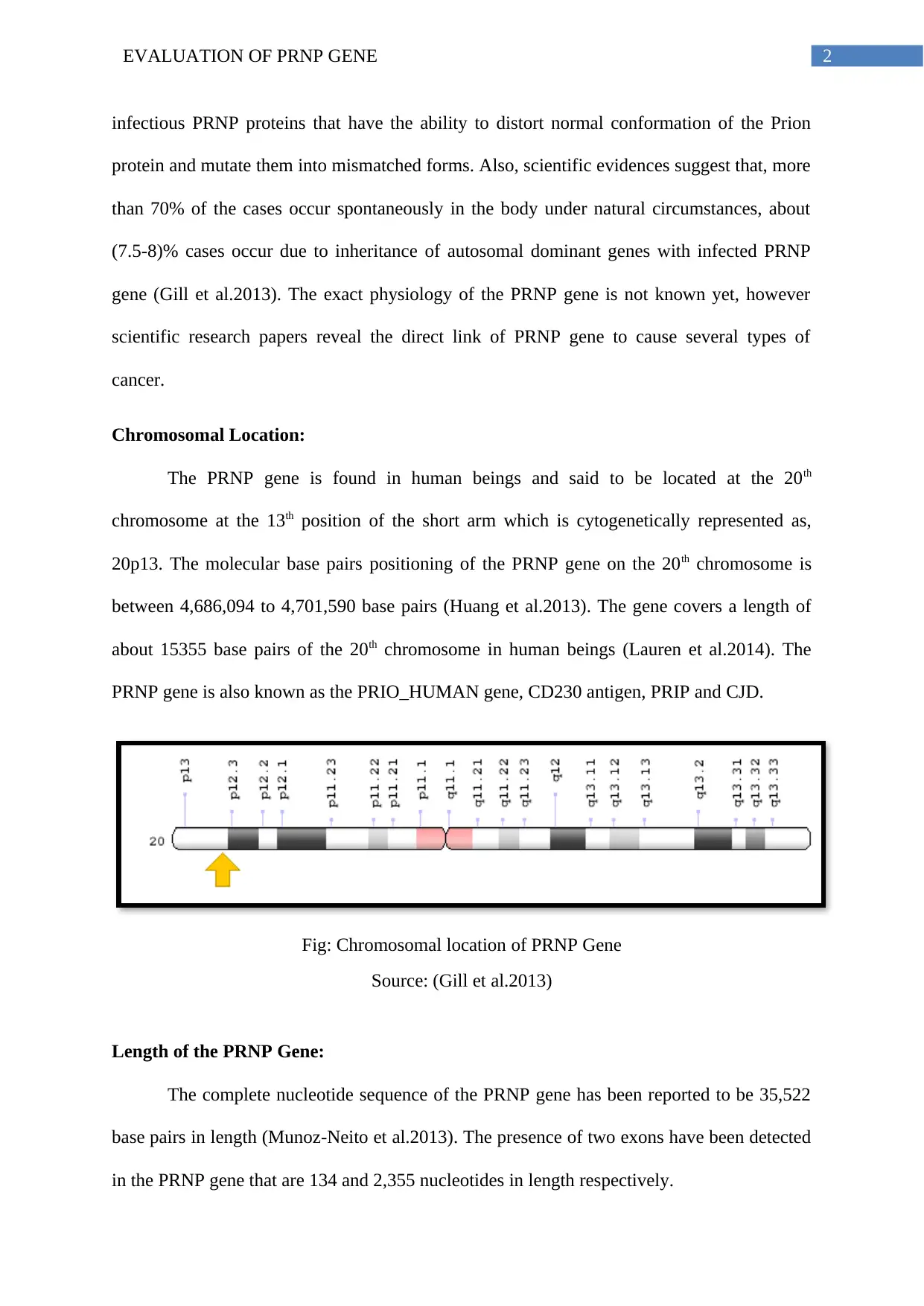
Chromosomal Location:
The PRNP gene is found in human beings and said to be located at the 20th
chromosome at the 13th position of the short arm which is cytogenetically represented as,
20p13. The molecular base pairs positioning of the PRNP gene on the 20th chromosome is
between 4,686,094 to 4,701,590 base pairs (Huang et al.2013). The gene covers a length of
about 15355 base pairs of the 20th chromosome in human beings (Lauren et al.2014). The
PRNP gene is also known as the PRIO_HUMAN gene, CD230 antigen, PRIP and CJD.
Fig: Chromosomal location of PRNP Gene
Source: (Gill et al.2013)
Length of the PRNP Gene:
The complete nucleotide sequence of the PRNP gene has been reported to be 35,522
base pairs in length (Munoz-Neito et al.2013). The presence of two exons have been detected
in the PRNP gene that are 134 and 2,355 nucleotides in length respectively.
infectious PRNP proteins that have the ability to distort normal conformation of the Prion
protein and mutate them into mismatched forms. Also, scientific evidences suggest that, more
than 70% of the cases occur spontaneously in the body under natural circumstances, about
(7.5-8)% cases occur due to inheritance of autosomal dominant genes with infected PRNP
gene (Gill et al.2013). The exact physiology of the PRNP gene is not known yet, however
scientific research papers reveal the direct link of PRNP gene to cause several types of
cancer.
Chromosomal Location:
The PRNP gene is found in human beings and said to be located at the 20th
chromosome at the 13th position of the short arm which is cytogenetically represented as,
20p13. The molecular base pairs positioning of the PRNP gene on the 20th chromosome is
between 4,686,094 to 4,701,590 base pairs (Huang et al.2013). The gene covers a length of
about 15355 base pairs of the 20th chromosome in human beings (Lauren et al.2014). The
PRNP gene is also known as the PRIO_HUMAN gene, CD230 antigen, PRIP and CJD.
Fig: Chromosomal location of PRNP Gene
Source: (Gill et al.2013)
Length of the PRNP Gene:
The complete nucleotide sequence of the PRNP gene has been reported to be 35,522
base pairs in length (Munoz-Neito et al.2013). The presence of two exons have been detected
in the PRNP gene that are 134 and 2,355 nucleotides in length respectively.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3EVALUATION OF PRNP GENE
Fig: Genetic sequence of PRION Gene
Source: (Uniprot.org. 2018. UniProt)
Presence of Introns:
The two exons are separated by an intron of 12,696 nitrogenous bases.
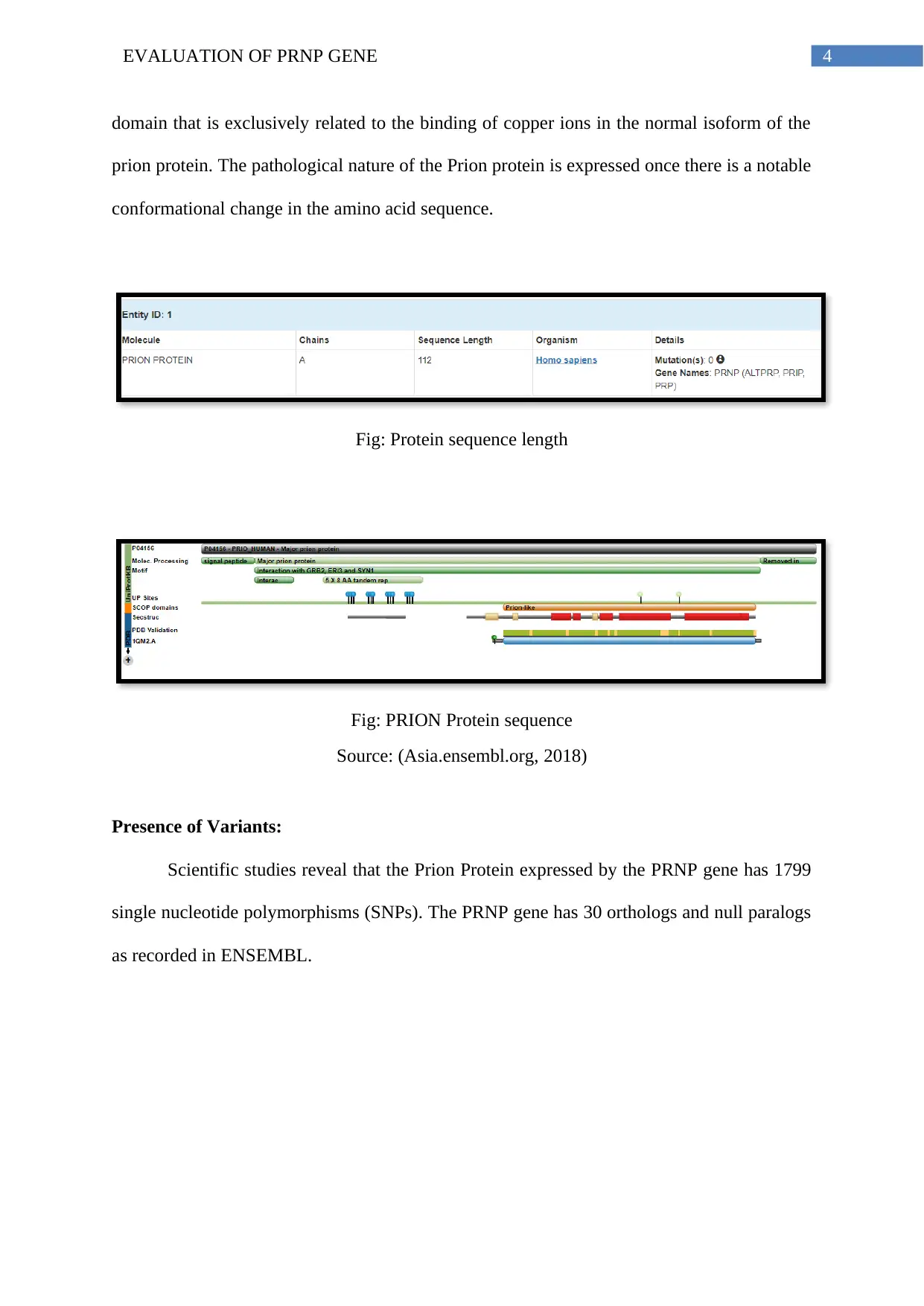
Length of the Protein:
The Prion protein sequence length in human being is found to be 112 base pairs long.
It should be noted here that there has been a documented similarity of about 97% between the
amino acid sequence found in human species and that of primates. The structure of the Prion
protein encoded by the PRNP gene comprises of three alpha helical strands with two beta
sheets that are anti-parallel in alignment (Sano et al.2013). The protein possesses a globule
like domain and has a short carboxyl tail and a NH2 terminal tail. There is a presence of
glycophosphatidylinositol anchor like membrane that helps in the adherence of the carboxyl
terminal to the inside of the cell membranes and this is responsible for exhibiting any kind of
characteristic conformational change. Prior to post-translational mechanism the PRNP gene is
found to be made up of 253 amino acids. As a result of the signalling cascade, during the
post-translational mechanism the signal sequences from the amino and the carboxyl group is
spliced and the resultant amino acid of 208 amino acid is obtained (Sonati et al.2013). The
protein structure reveals of Prion protein reveals the presence of five amino-terminal octa-
peptide repeats with the constant sequence PHGGGWGQ, that is believed to give rise to a
Fig: Genetic sequence of PRION Gene
Source: (Uniprot.org. 2018. UniProt)
Presence of Introns:
The two exons are separated by an intron of 12,696 nitrogenous bases.
Length of the Protein:
The Prion protein sequence length in human being is found to be 112 base pairs long.
It should be noted here that there has been a documented similarity of about 97% between the
amino acid sequence found in human species and that of primates. The structure of the Prion
protein encoded by the PRNP gene comprises of three alpha helical strands with two beta
sheets that are anti-parallel in alignment (Sano et al.2013). The protein possesses a globule
like domain and has a short carboxyl tail and a NH2 terminal tail. There is a presence of
glycophosphatidylinositol anchor like membrane that helps in the adherence of the carboxyl
terminal to the inside of the cell membranes and this is responsible for exhibiting any kind of
characteristic conformational change. Prior to post-translational mechanism the PRNP gene is
found to be made up of 253 amino acids. As a result of the signalling cascade, during the
post-translational mechanism the signal sequences from the amino and the carboxyl group is
spliced and the resultant amino acid of 208 amino acid is obtained (Sonati et al.2013). The
protein structure reveals of Prion protein reveals the presence of five amino-terminal octa-
peptide repeats with the constant sequence PHGGGWGQ, that is believed to give rise to a
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
4EVALUATION OF PRNP GENE
domain that is exclusively related to the binding of copper ions in the normal isoform of the
prion protein. The pathological nature of the Prion protein is expressed once there is a notable
conformational change in the amino acid sequence.
Fig: Protein sequence length
Fig: PRION Protein sequence
Source: (Asia.ensembl.org, 2018)
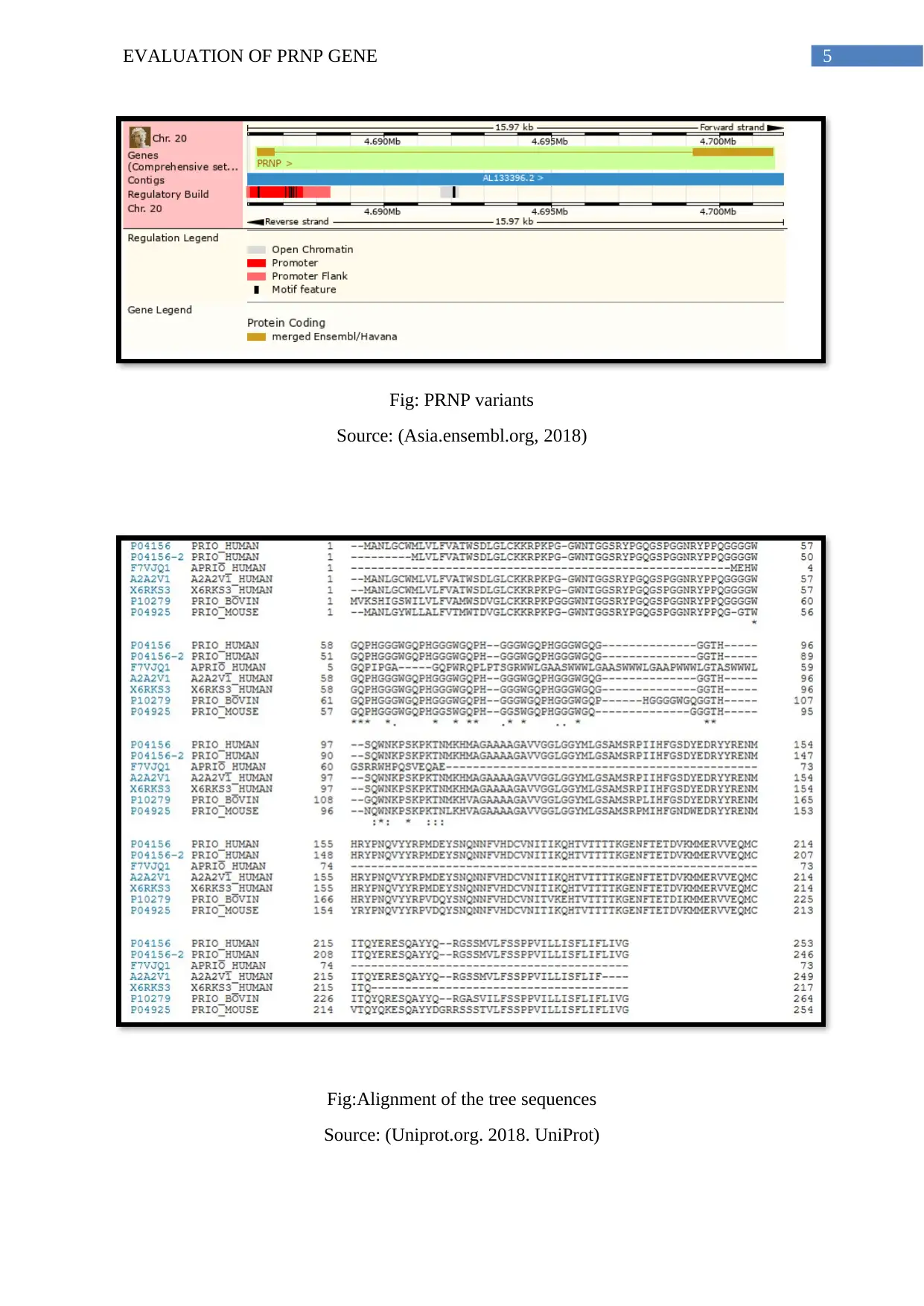
Presence of Variants:
Scientific studies reveal that the Prion Protein expressed by the PRNP gene has 1799
single nucleotide polymorphisms (SNPs). The PRNP gene has 30 orthologs and null paralogs
as recorded in ENSEMBL.
domain that is exclusively related to the binding of copper ions in the normal isoform of the
prion protein. The pathological nature of the Prion protein is expressed once there is a notable
conformational change in the amino acid sequence.
Fig: Protein sequence length
Fig: PRION Protein sequence
Source: (Asia.ensembl.org, 2018)
Presence of Variants:
Scientific studies reveal that the Prion Protein expressed by the PRNP gene has 1799
single nucleotide polymorphisms (SNPs). The PRNP gene has 30 orthologs and null paralogs
as recorded in ENSEMBL.
5EVALUATION OF PRNP GENE
Fig: PRNP variants
Source: (Asia.ensembl.org, 2018)
Fig:Alignment of the tree sequences
Source: (Uniprot.org. 2018. UniProt)
Fig: PRNP variants
Source: (Asia.ensembl.org, 2018)
Fig:Alignment of the tree sequences
Source: (Uniprot.org. 2018. UniProt)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6EVALUATION OF PRNP GENE
The above figure represents the alignment of the PRNP protein in human, bovine and
mouse species using UniProt database.
Fig:PRNP Model
Source: (Uniprot.org. 2018. UniProt)
The above figure represents the alignment of the PRNP protein in human, bovine and
mouse species using UniProt database.
Fig:PRNP Model
Source: (Uniprot.org. 2018. UniProt)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
7EVALUATION OF PRNP GENE
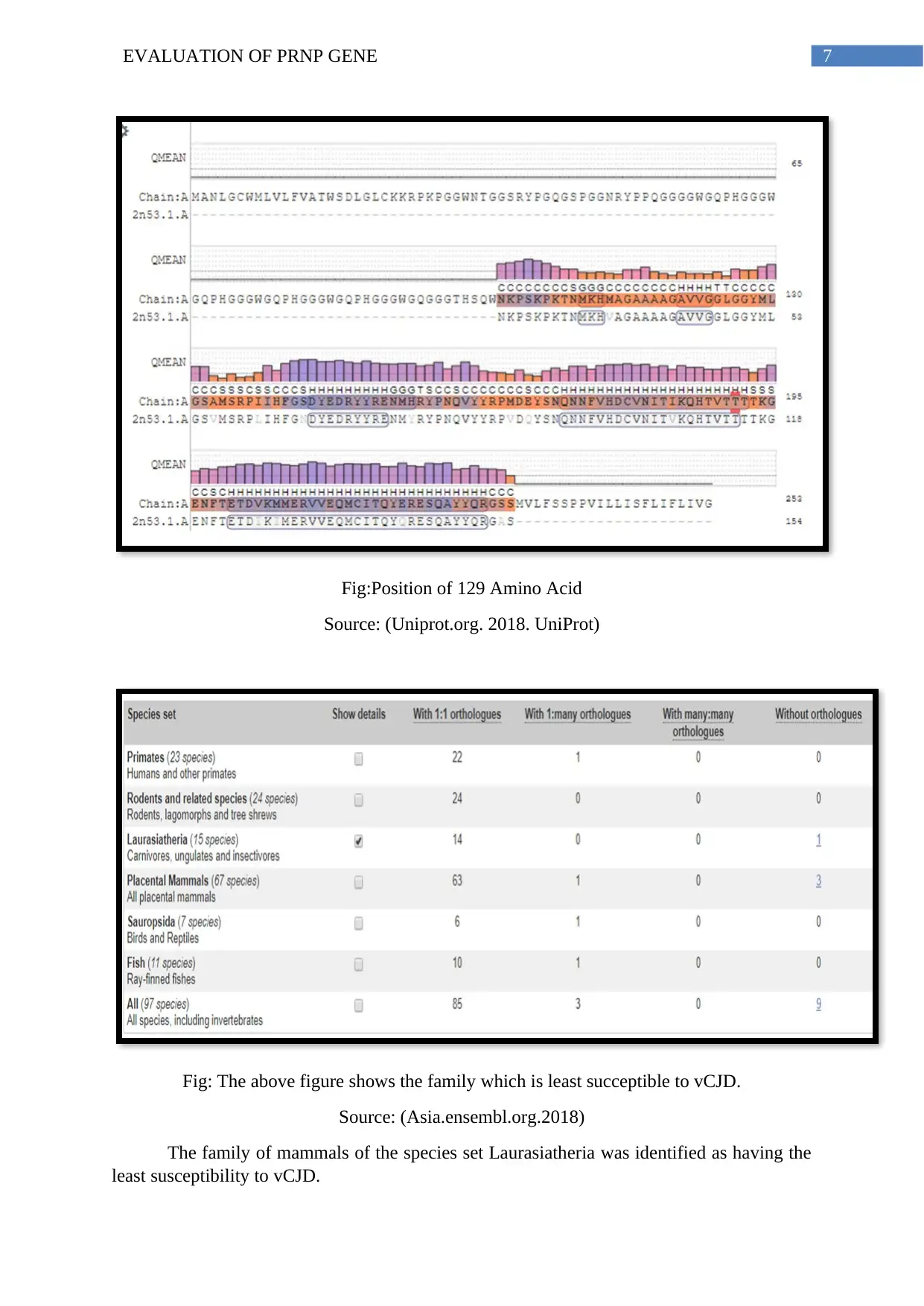
Fig:Position of 129 Amino Acid
Source: (Uniprot.org. 2018. UniProt)
Fig: The above figure shows the family which is least succeptible to vCJD.
Source: (Asia.ensembl.org.2018)
The family of mammals of the species set Laurasiatheria was identified as having the
least susceptibility to vCJD.
Fig:Position of 129 Amino Acid
Source: (Uniprot.org. 2018. UniProt)
Fig: The above figure shows the family which is least succeptible to vCJD.
Source: (Asia.ensembl.org.2018)
The family of mammals of the species set Laurasiatheria was identified as having the
least susceptibility to vCJD.

8EVALUATION OF PRNP GENE
Conclusion:
Thus, the report presents a crystal clear over-view on the PRNP gene in human beings
and also addresses all the questions, pertaining to the chromosomal structure, function,
variant, exons, introns and protein structure of the gene in an efficient way, making use of the
bioinformatic databases which includes the use of ENSEMBL, uniProt and SwissProt. The
PRNP gene is sensitive and the slightest of conformational change can lead to the cause of a
deformity.
Conclusion:
Thus, the report presents a crystal clear over-view on the PRNP gene in human beings
and also addresses all the questions, pertaining to the chromosomal structure, function,
variant, exons, introns and protein structure of the gene in an efficient way, making use of the
bioinformatic databases which includes the use of ENSEMBL, uniProt and SwissProt. The
PRNP gene is sensitive and the slightest of conformational change can lead to the cause of a
deformity.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9EVALUATION OF PRNP GENE
References:
Asante, E. A., Smidak, M., Grimshaw, A., Houghton, R., Tomlinson, A., Jeelani, A. and
Brandner, S. 2015. A naturally occurring variant of the human prion protein completely
prevents prion disease. Nature, 522(7557), 478.
Asia.ensembl.org. 2018. Ensembl genome browser 92. [online] Available at:
https://asia.ensembl.org/index.html [Accessed 16 Jul. 2018].
Brown, D.R., Qin, K., Herms, J.W., Madlung, A., Manson, J., Strome, R., Fraser, P.E.,
Kruck, T., von Bohlen, A., Schulz-Schaeffer, W. and Giese, A., 2014. The cellular prion
protein binds copper in vivo. Nature, 390(6661), p.684.
Cordeiro, Y., Machado, F., Neto, L.J., Juliano, M.A., Brentani, R.R., Foguel, D. and Silva,
J.L., 2012. DNA converts cellular prion protein into the beta-sheet conformation and inhibits
prion peptide aggregation. Journal of Biological Chemistry.
Fluharty, B. R., Biasini, E., Stravalaci, M., Sclip, A., Diomede, L., Balducci, C. and Borsello,
T. 2013. An N-terminal fragment of the prion protein binds to amyloid-β oligomers and
inhibits their neurotoxicity in vivo. Journal of Biological Chemistry, jbc-M112.
Gill, O. N., Spencer, Y., Richard-Loendt, A., Kelly, C., Dabaghian, R., Boyes, L. and
Andrews, N. 2013. Prevalent abnormal prion protein in human appendixes after bovine
spongiform encephalopathy epizootic: large scale survey. Bmj, 347, f5675.
Huang, Z., Gabriel, J.M., Baldwin, M.A., Fletterick, R.J., Prusiner, S.B. and Cohen, F.E.,
2013. Proposed three-dimensional structure for the cellular prion protein. Proceedings of the
National Academy of Sciences, 91(15), pp.7139-7143.
References:
Asante, E. A., Smidak, M., Grimshaw, A., Houghton, R., Tomlinson, A., Jeelani, A. and
Brandner, S. 2015. A naturally occurring variant of the human prion protein completely
prevents prion disease. Nature, 522(7557), 478.
Asia.ensembl.org. 2018. Ensembl genome browser 92. [online] Available at:
https://asia.ensembl.org/index.html [Accessed 16 Jul. 2018].
Brown, D.R., Qin, K., Herms, J.W., Madlung, A., Manson, J., Strome, R., Fraser, P.E.,
Kruck, T., von Bohlen, A., Schulz-Schaeffer, W. and Giese, A., 2014. The cellular prion
protein binds copper in vivo. Nature, 390(6661), p.684.
Cordeiro, Y., Machado, F., Neto, L.J., Juliano, M.A., Brentani, R.R., Foguel, D. and Silva,
J.L., 2012. DNA converts cellular prion protein into the beta-sheet conformation and inhibits
prion peptide aggregation. Journal of Biological Chemistry.
Fluharty, B. R., Biasini, E., Stravalaci, M., Sclip, A., Diomede, L., Balducci, C. and Borsello,
T. 2013. An N-terminal fragment of the prion protein binds to amyloid-β oligomers and
inhibits their neurotoxicity in vivo. Journal of Biological Chemistry, jbc-M112.
Gill, O. N., Spencer, Y., Richard-Loendt, A., Kelly, C., Dabaghian, R., Boyes, L. and
Andrews, N. 2013. Prevalent abnormal prion protein in human appendixes after bovine
spongiform encephalopathy epizootic: large scale survey. Bmj, 347, f5675.
Huang, Z., Gabriel, J.M., Baldwin, M.A., Fletterick, R.J., Prusiner, S.B. and Cohen, F.E.,
2013. Proposed three-dimensional structure for the cellular prion protein. Proceedings of the
National Academy of Sciences, 91(15), pp.7139-7143.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10EVALUATION OF PRNP GENE
Laurén, J., Gimbel, D.A., Nygaard, H.B., Gilbert, J.W. and Strittmatter, S.M., 2014. Cellular
prion protein mediates impairment of synaptic plasticity by amyloid-β
oligomers. Nature, 457(7233), p.1128.
Muñoz-Nieto, M., Ramonet, N., López-Gastón, J. I., Cuadrado-Corrales, N., Calero, O.,
Díaz-Hurtado, M., ... and Calero, M. 2013. A novel mutation I215V in the PRNP gene
associated with Creutzfeldt–Jakob and Alzheimer’s diseases in three patients with divergent
clinical phenotypes. Journal of neurology, 260(1), 77-84.
Sano, K., Satoh, K., Atarashi, R., Takashima, H., Iwasaki, Y., Yoshida, M., and Zerr, I. 2013.
Early detection of abnormal prion protein in genetic human prion diseases now possible using
real-time QUIC assay. PloS one, 8(1), e54915.
Sonati, T., Reimann, R. R., Falsig, J., Baral, P. K., O’connor, T., Hornemann, S. and
Swayampakula, M. 2013. The toxicity of antiprion antibodies is mediated by the flexible tail
of the prion protein. Nature, 501(7465), 102.
Uniprot.org. 2018. UniProt. [online] Available at: https://www.uniprot.org/ [Accessed 16 Jul.
2018].
Laurén, J., Gimbel, D.A., Nygaard, H.B., Gilbert, J.W. and Strittmatter, S.M., 2014. Cellular
prion protein mediates impairment of synaptic plasticity by amyloid-β
oligomers. Nature, 457(7233), p.1128.
Muñoz-Nieto, M., Ramonet, N., López-Gastón, J. I., Cuadrado-Corrales, N., Calero, O.,
Díaz-Hurtado, M., ... and Calero, M. 2013. A novel mutation I215V in the PRNP gene
associated with Creutzfeldt–Jakob and Alzheimer’s diseases in three patients with divergent
clinical phenotypes. Journal of neurology, 260(1), 77-84.
Sano, K., Satoh, K., Atarashi, R., Takashima, H., Iwasaki, Y., Yoshida, M., and Zerr, I. 2013.
Early detection of abnormal prion protein in genetic human prion diseases now possible using
real-time QUIC assay. PloS one, 8(1), e54915.
Sonati, T., Reimann, R. R., Falsig, J., Baral, P. K., O’connor, T., Hornemann, S. and
Swayampakula, M. 2013. The toxicity of antiprion antibodies is mediated by the flexible tail
of the prion protein. Nature, 501(7465), 102.
Uniprot.org. 2018. UniProt. [online] Available at: https://www.uniprot.org/ [Accessed 16 Jul.
2018].
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





