Macquarie University: Protein Study - Growth Hormone Analysis
VerifiedAdded on 2022/07/28
|9
|1498
|25
Report
AI Summary
This report provides a comprehensive analysis of growth hormone (somatotropin), a 190-amino acid protein synthesized in the pituitary gland. It begins by detailing the primary structure, including the amino acid sequence, signal peptide, mature peptide chain, and disulfide bonds. The report then delves into the physico-chemical properties of the protein, such as its mature peptide sequence, amino acid composition, molecular weight, theoretical pI, and atom composition. A hydropathy plot is presented and discussed to measure the hydrophobicity and hydrophilicity of the amino acids. The report further examines the historical purification steps and the present purification process, including anion exchange chromatography and gel filtration chromatography. The report also includes a bibliography of relevant sources.

Running head: PROTEIN DISCOVERY AND ANALYSIS
GROWTH HORMONE
Name of the Student
Name of the University
Author Note
GROWTH HORMONE
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
Table of Contents
Introduction................................................................................................................................3
The primary structure:................................................................................................................3
Physico-chemical properties of the protein:...............................................................................3
Matured peptide sequence......................................................................................................3
Amino acid composition with pI and MW.............................................................................4
Hydropathy plot.....................................................................................................................5
Historical purification step.........................................................................................................7
Present purification process.......................................................................................................8
Bibliography...............................................................................................................................9
Table of Contents
Introduction................................................................................................................................3
The primary structure:................................................................................................................3
Physico-chemical properties of the protein:...............................................................................3
Matured peptide sequence......................................................................................................3
Amino acid composition with pI and MW.............................................................................4
Hydropathy plot.....................................................................................................................5
Historical purification step.........................................................................................................7
Present purification process.......................................................................................................8
Bibliography...............................................................................................................................9
3
Introduction
Growth hormone has been stated as the protein hormone which is secreted as 190 amino acid
containing protein. This hormone is synthesized from pituitary cells known as Somatotrophs.
This hormone us a major participant of various complex process of human physiology
including both metabolism and growth. This paper will discuss the primary structure,
physico-chemical properties and purification processes of growth hormone (somatotropin).
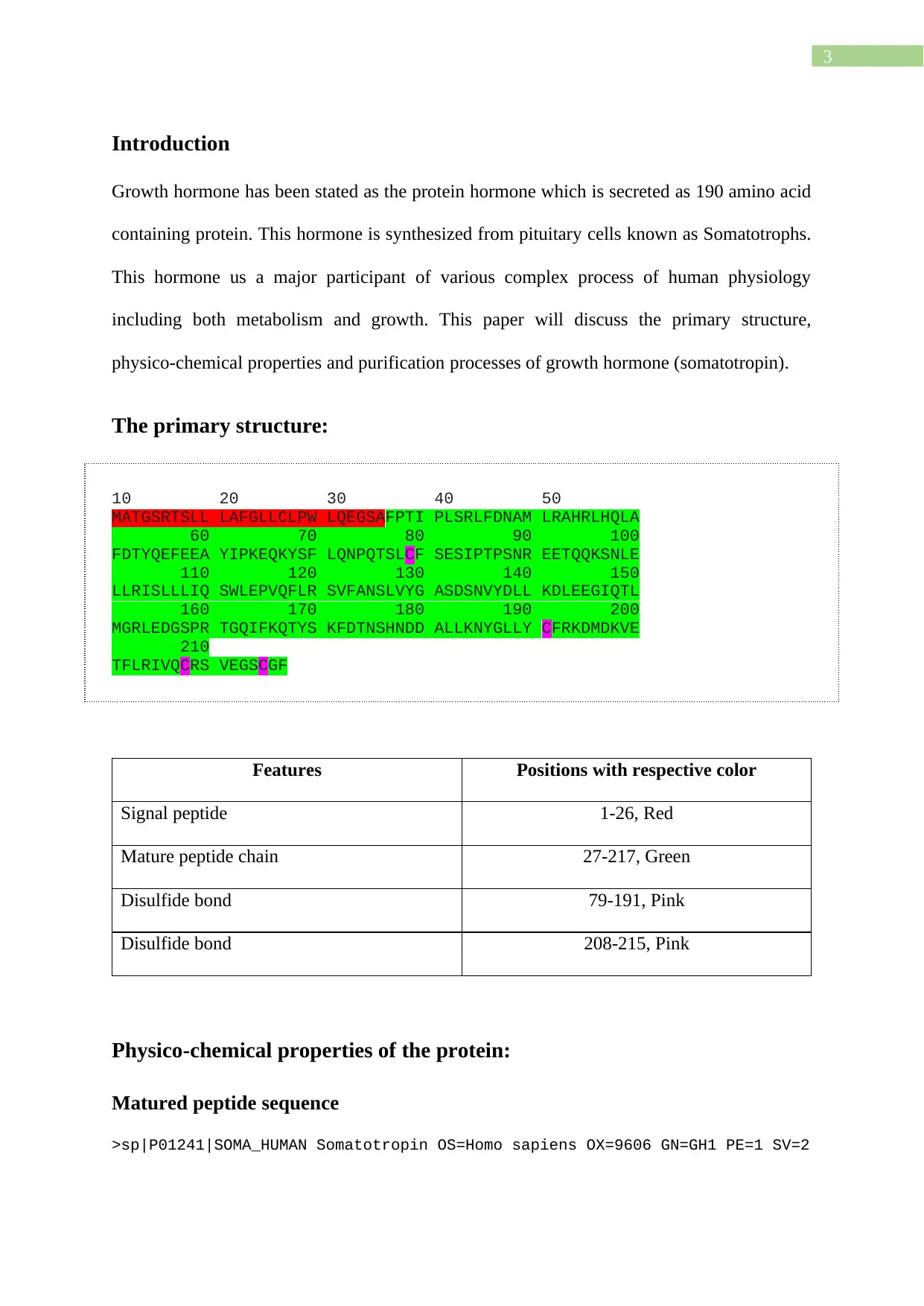
The primary structure:
10 20 30 40 50
MATGSRTSLL LAFGLLCLPW LQEGSAFPTI PLSRLFDNAM LRAHRLHQLA
60 70 80 90 100
FDTYQEFEEA YIPKEQKYSF LQNPQTSLCF SESIPTPSNR EETQQKSNLE
110 120 130 140 150
LLRISLLLIQ SWLEPVQFLR SVFANSLVYG ASDSNVYDLL KDLEEGIQTL
160 170 180 190 200
MGRLEDGSPR TGQIFKQTYS KFDTNSHNDD ALLKNYGLLY CFRKDMDKVE
210
TFLRIVQCRS VEGSCGF
Features Positions with respective color
Signal peptide 1-26, Red
Mature peptide chain 27-217, Green
Disulfide bond 79-191, Pink
Disulfide bond 208-215, Pink
Physico-chemical properties of the protein:
Matured peptide sequence
>sp|P01241|SOMA_HUMAN Somatotropin OS=Homo sapiens OX=9606 GN=GH1 PE=1 SV=2
Introduction
Growth hormone has been stated as the protein hormone which is secreted as 190 amino acid
containing protein. This hormone is synthesized from pituitary cells known as Somatotrophs.
This hormone us a major participant of various complex process of human physiology
including both metabolism and growth. This paper will discuss the primary structure,
physico-chemical properties and purification processes of growth hormone (somatotropin).
The primary structure:
10 20 30 40 50
MATGSRTSLL LAFGLLCLPW LQEGSAFPTI PLSRLFDNAM LRAHRLHQLA
60 70 80 90 100
FDTYQEFEEA YIPKEQKYSF LQNPQTSLCF SESIPTPSNR EETQQKSNLE
110 120 130 140 150
LLRISLLLIQ SWLEPVQFLR SVFANSLVYG ASDSNVYDLL KDLEEGIQTL
160 170 180 190 200
MGRLEDGSPR TGQIFKQTYS KFDTNSHNDD ALLKNYGLLY CFRKDMDKVE
210
TFLRIVQCRS VEGSCGF
Features Positions with respective color
Signal peptide 1-26, Red
Mature peptide chain 27-217, Green
Disulfide bond 79-191, Pink
Disulfide bond 208-215, Pink
Physico-chemical properties of the protein:
Matured peptide sequence
>sp|P01241|SOMA_HUMAN Somatotropin OS=Homo sapiens OX=9606 GN=GH1 PE=1 SV=2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
4
FPTIPLSRLFDNAMLRAHRLHQLAFDTYQEFEEAYIPKEQKYSFLQNPQTSLCFSESIPTPSNREETQQKSNLEL
LRISLLLIQSWLEPVQFLRSVFANSLVYGASDSNVYDLLKDLEEGIQTLMGRLEDGSPRTGQIFKQTYSKFDTNS
HNDDALLKNYGLLYCFRKDMDKVETFLRIVQCRSVEGSCGF
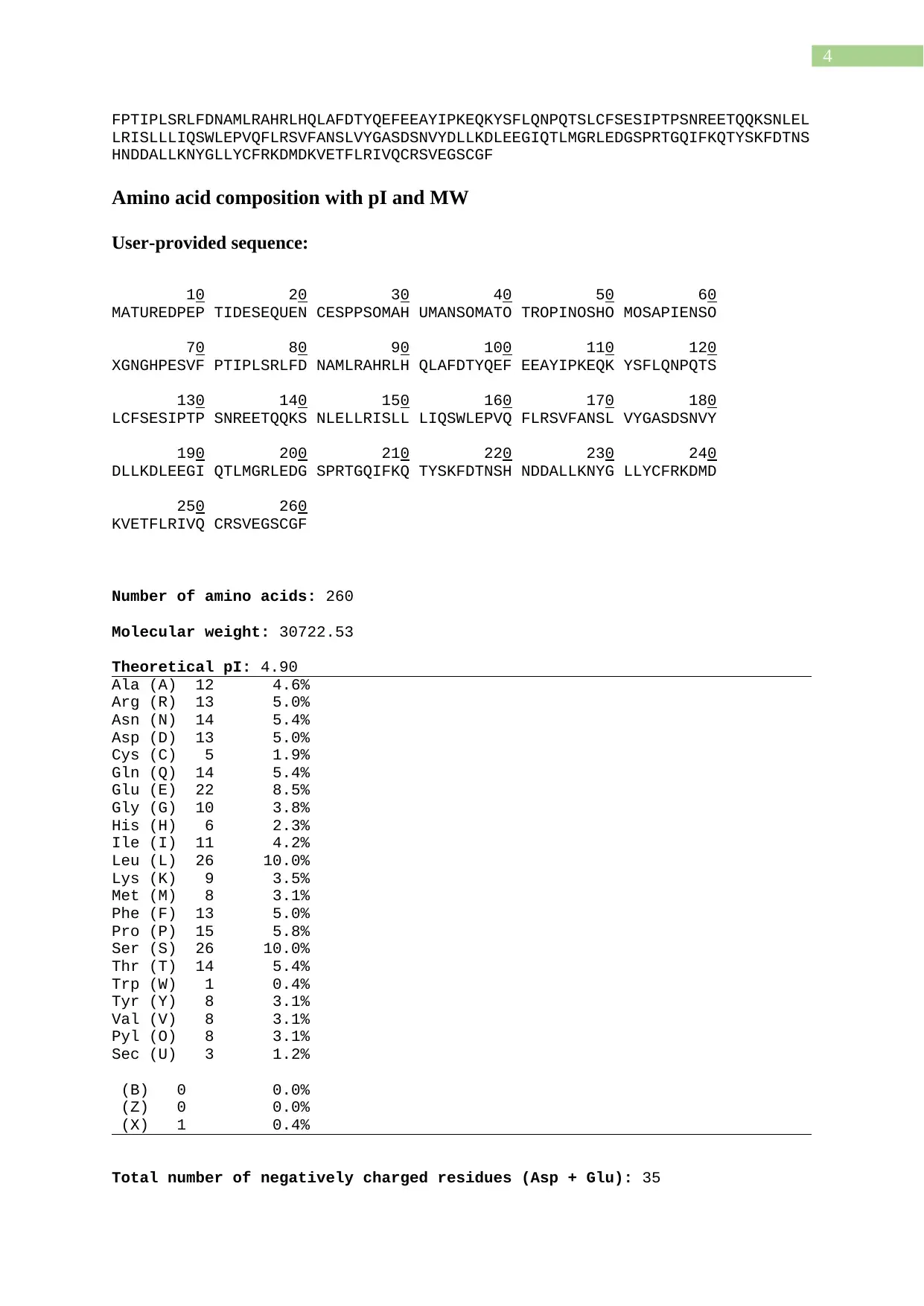
Amino acid composition with pI and MW
User-provided sequence:
10 20 30 40 50 60
MATUREDPEP TIDESEQUEN CESPPSOMAH UMANSOMATO TROPINOSHO MOSAPIENSO
70 80 90 100 110 120
XGNGHPESVF PTIPLSRLFD NAMLRAHRLH QLAFDTYQEF EEAYIPKEQK YSFLQNPQTS
130 140 150 160 170 180
LCFSESIPTP SNREETQQKS NLELLRISLL LIQSWLEPVQ FLRSVFANSL VYGASDSNVY
190 200 210 220 230 240
DLLKDLEEGI QTLMGRLEDG SPRTGQIFKQ TYSKFDTNSH NDDALLKNYG LLYCFRKDMD
250 260
KVETFLRIVQ CRSVEGSCGF
Number of amino acids: 260
Molecular weight: 30722.53
Theoretical pI: 4.90
Ala (A) 12 4.6%
Arg (R) 13 5.0%
Asn (N) 14 5.4%
Asp (D) 13 5.0%
Cys (C) 5 1.9%
Gln (Q) 14 5.4%
Glu (E) 22 8.5%
Gly (G) 10 3.8%
His (H) 6 2.3%
Ile (I) 11 4.2%
Leu (L) 26 10.0%
Lys (K) 9 3.5%
Met (M) 8 3.1%
Phe (F) 13 5.0%
Pro (P) 15 5.8%
Ser (S) 26 10.0%
Thr (T) 14 5.4%
Trp (W) 1 0.4%
Tyr (Y) 8 3.1%
Val (V) 8 3.1%
Pyl (O) 8 3.1%
Sec (U) 3 1.2%
(B) 0 0.0%
(Z) 0 0.0%
(X) 1 0.4%
Total number of negatively charged residues (Asp + Glu): 35
FPTIPLSRLFDNAMLRAHRLHQLAFDTYQEFEEAYIPKEQKYSFLQNPQTSLCFSESIPTPSNREETQQKSNLEL
LRISLLLIQSWLEPVQFLRSVFANSLVYGASDSNVYDLLKDLEEGIQTLMGRLEDGSPRTGQIFKQTYSKFDTNS
HNDDALLKNYGLLYCFRKDMDKVETFLRIVQCRSVEGSCGF
Amino acid composition with pI and MW
User-provided sequence:
10 20 30 40 50 60
MATUREDPEP TIDESEQUEN CESPPSOMAH UMANSOMATO TROPINOSHO MOSAPIENSO
70 80 90 100 110 120
XGNGHPESVF PTIPLSRLFD NAMLRAHRLH QLAFDTYQEF EEAYIPKEQK YSFLQNPQTS
130 140 150 160 170 180
LCFSESIPTP SNREETQQKS NLELLRISLL LIQSWLEPVQ FLRSVFANSL VYGASDSNVY
190 200 210 220 230 240
DLLKDLEEGI QTLMGRLEDG SPRTGQIFKQ TYSKFDTNSH NDDALLKNYG LLYCFRKDMD
250 260
KVETFLRIVQ CRSVEGSCGF
Number of amino acids: 260
Molecular weight: 30722.53
Theoretical pI: 4.90
Ala (A) 12 4.6%
Arg (R) 13 5.0%
Asn (N) 14 5.4%
Asp (D) 13 5.0%
Cys (C) 5 1.9%
Gln (Q) 14 5.4%
Glu (E) 22 8.5%
Gly (G) 10 3.8%
His (H) 6 2.3%
Ile (I) 11 4.2%
Leu (L) 26 10.0%
Lys (K) 9 3.5%
Met (M) 8 3.1%
Phe (F) 13 5.0%
Pro (P) 15 5.8%
Ser (S) 26 10.0%
Thr (T) 14 5.4%
Trp (W) 1 0.4%
Tyr (Y) 8 3.1%
Val (V) 8 3.1%
Pyl (O) 8 3.1%
Sec (U) 3 1.2%
(B) 0 0.0%
(Z) 0 0.0%
(X) 1 0.4%
Total number of negatively charged residues (Asp + Glu): 35
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
5
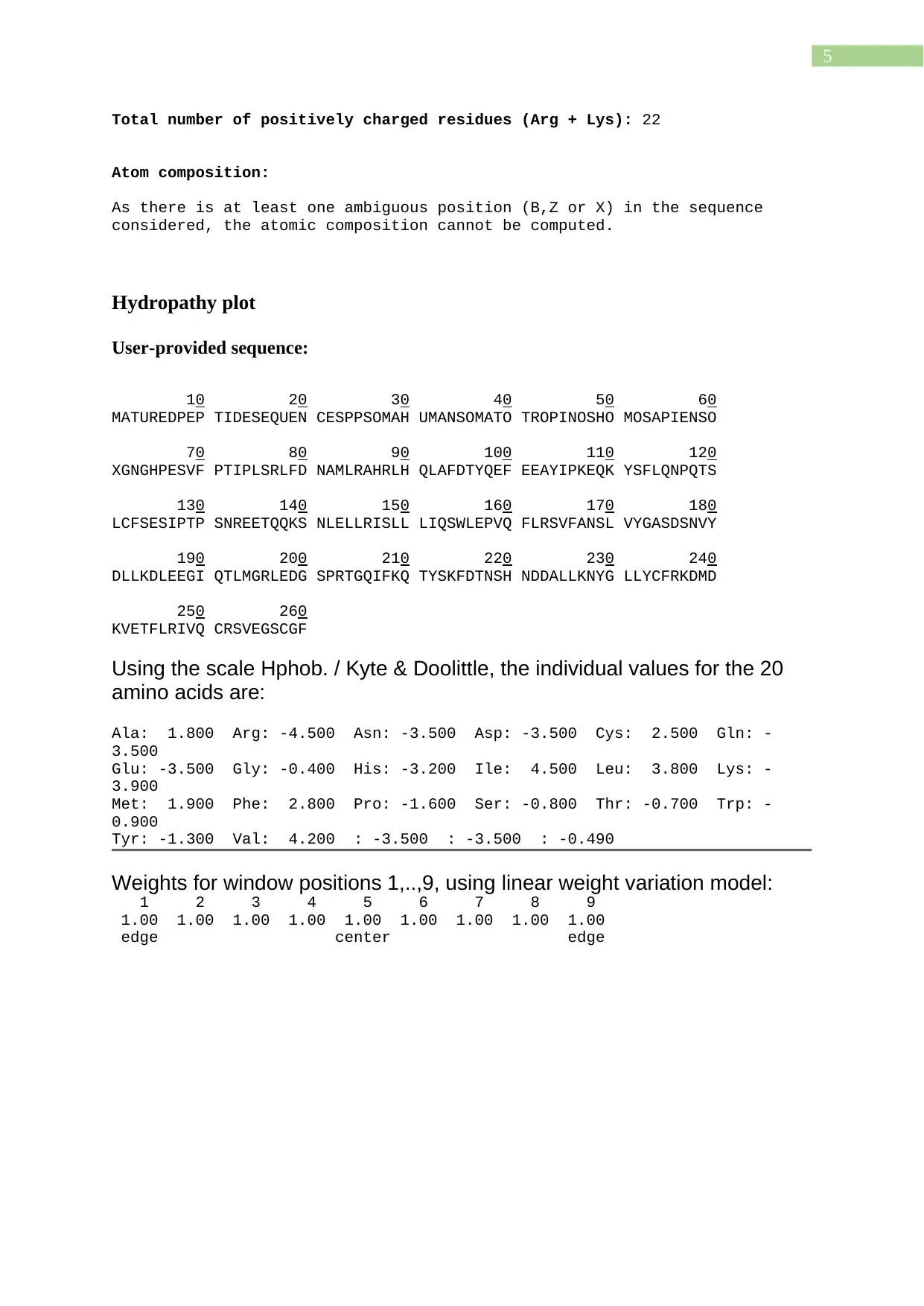
Total number of positively charged residues (Arg + Lys): 22
Atom composition:
As there is at least one ambiguous position (B,Z or X) in the sequence
considered, the atomic composition cannot be computed.
Hydropathy plot
User-provided sequence:
10 20 30 40 50 60
MATUREDPEP TIDESEQUEN CESPPSOMAH UMANSOMATO TROPINOSHO MOSAPIENSO
70 80 90 100 110 120
XGNGHPESVF PTIPLSRLFD NAMLRAHRLH QLAFDTYQEF EEAYIPKEQK YSFLQNPQTS
130 140 150 160 170 180
LCFSESIPTP SNREETQQKS NLELLRISLL LIQSWLEPVQ FLRSVFANSL VYGASDSNVY
190 200 210 220 230 240
DLLKDLEEGI QTLMGRLEDG SPRTGQIFKQ TYSKFDTNSH NDDALLKNYG LLYCFRKDMD
250 260
KVETFLRIVQ CRSVEGSCGF
Using the scale Hphob. / Kyte & Doolittle, the individual values for the 20
amino acids are:
Ala: 1.800 Arg: -4.500 Asn: -3.500 Asp: -3.500 Cys: 2.500 Gln: -
3.500
Glu: -3.500 Gly: -0.400 His: -3.200 Ile: 4.500 Leu: 3.800 Lys: -
3.900
Met: 1.900 Phe: 2.800 Pro: -1.600 Ser: -0.800 Thr: -0.700 Trp: -
0.900
Tyr: -1.300 Val: 4.200 : -3.500 : -3.500 : -0.490
Weights for window positions 1,..,9, using linear weight variation model:
1 2 3 4 5 6 7 8 9
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00
edge center edge
Total number of positively charged residues (Arg + Lys): 22
Atom composition:
As there is at least one ambiguous position (B,Z or X) in the sequence
considered, the atomic composition cannot be computed.
Hydropathy plot
User-provided sequence:
10 20 30 40 50 60
MATUREDPEP TIDESEQUEN CESPPSOMAH UMANSOMATO TROPINOSHO MOSAPIENSO
70 80 90 100 110 120
XGNGHPESVF PTIPLSRLFD NAMLRAHRLH QLAFDTYQEF EEAYIPKEQK YSFLQNPQTS
130 140 150 160 170 180
LCFSESIPTP SNREETQQKS NLELLRISLL LIQSWLEPVQ FLRSVFANSL VYGASDSNVY
190 200 210 220 230 240
DLLKDLEEGI QTLMGRLEDG SPRTGQIFKQ TYSKFDTNSH NDDALLKNYG LLYCFRKDMD
250 260
KVETFLRIVQ CRSVEGSCGF
Using the scale Hphob. / Kyte & Doolittle, the individual values for the 20
amino acids are:
Ala: 1.800 Arg: -4.500 Asn: -3.500 Asp: -3.500 Cys: 2.500 Gln: -
3.500
Glu: -3.500 Gly: -0.400 His: -3.200 Ile: 4.500 Leu: 3.800 Lys: -
3.900
Met: 1.900 Phe: 2.800 Pro: -1.600 Ser: -0.800 Thr: -0.700 Trp: -
0.900
Tyr: -1.300 Val: 4.200 : -3.500 : -3.500 : -0.490
Weights for window positions 1,..,9, using linear weight variation model:
1 2 3 4 5 6 7 8 9
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00
edge center edge
6
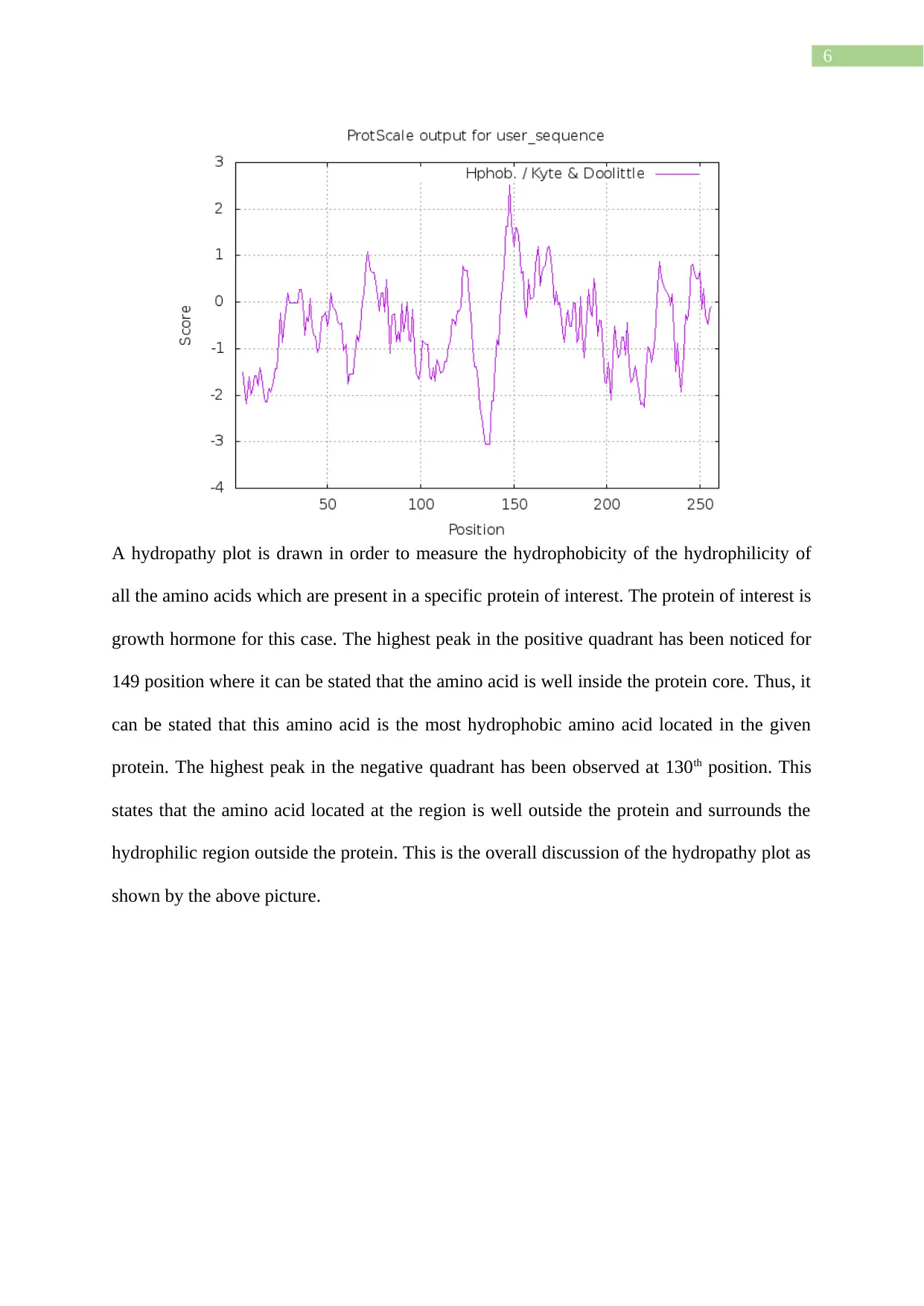
A hydropathy plot is drawn in order to measure the hydrophobicity of the hydrophilicity of
all the amino acids which are present in a specific protein of interest. The protein of interest is
growth hormone for this case. The highest peak in the positive quadrant has been noticed for
149 position where it can be stated that the amino acid is well inside the protein core. Thus, it
can be stated that this amino acid is the most hydrophobic amino acid located in the given
protein. The highest peak in the negative quadrant has been observed at 130th position. This
states that the amino acid located at the region is well outside the protein and surrounds the
hydrophilic region outside the protein. This is the overall discussion of the hydropathy plot as
shown by the above picture.
A hydropathy plot is drawn in order to measure the hydrophobicity of the hydrophilicity of
all the amino acids which are present in a specific protein of interest. The protein of interest is
growth hormone for this case. The highest peak in the positive quadrant has been noticed for
149 position where it can be stated that the amino acid is well inside the protein core. Thus, it
can be stated that this amino acid is the most hydrophobic amino acid located in the given
protein. The highest peak in the negative quadrant has been observed at 130th position. This
states that the amino acid located at the region is well outside the protein and surrounds the
hydrophilic region outside the protein. This is the overall discussion of the hydropathy plot as
shown by the above picture.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
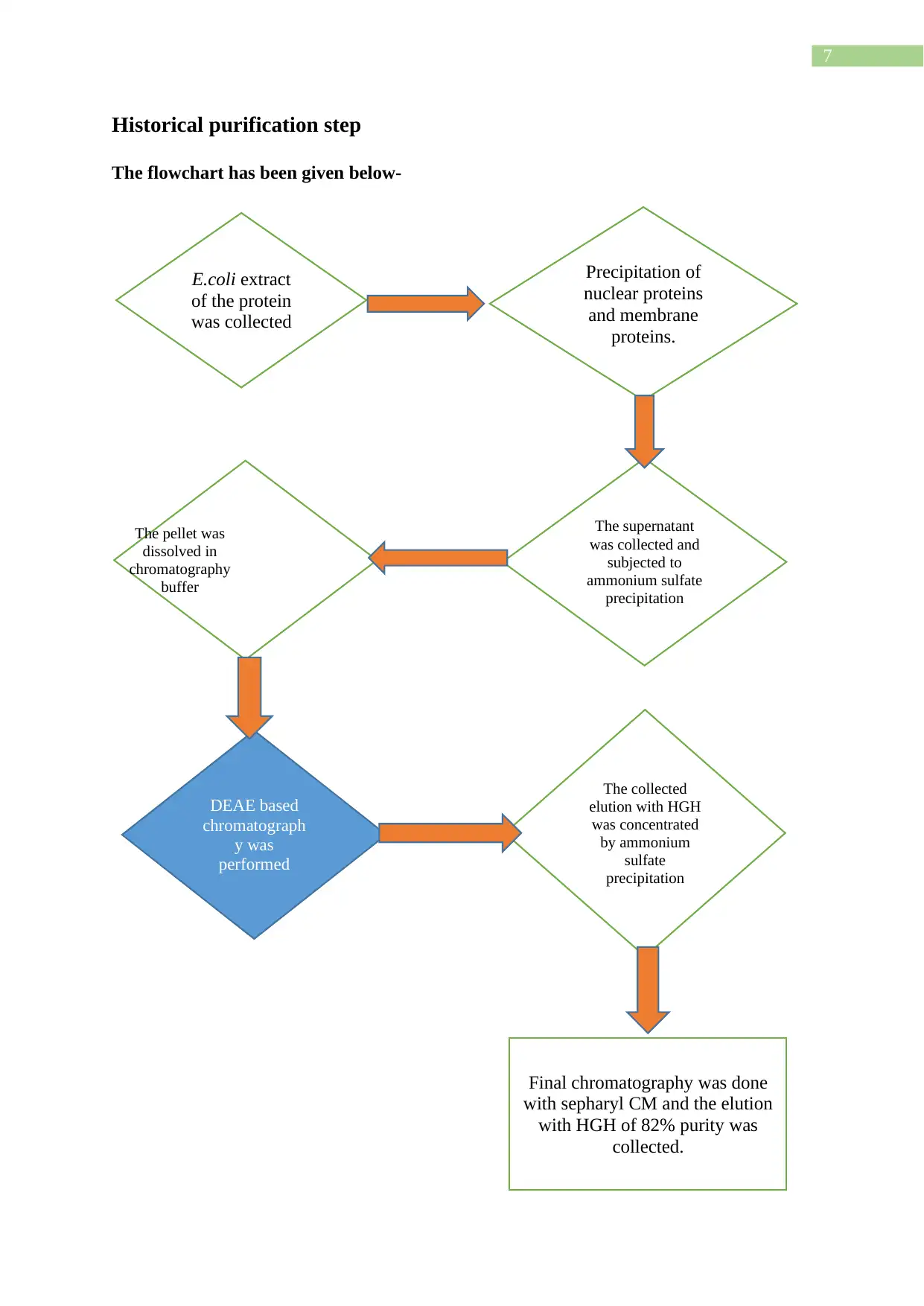
7
Historical purification step
The flowchart has been given below-
E.coli extract
of the protein
was collected
Precipitation of
nuclear proteins
and membrane
proteins.
The supernatant
was collected and
subjected to
ammonium sulfate
precipitation
The pellet was
dissolved in
chromatography
buffer
The collected
elution with HGH
was concentrated
by ammonium
sulfate
precipitation
DEAE based
chromatograph
y was
performed
Final chromatography was done
with sepharyl CM and the elution
with HGH of 82% purity was
collected.
Historical purification step
The flowchart has been given below-
E.coli extract
of the protein
was collected
Precipitation of
nuclear proteins
and membrane
proteins.
The supernatant
was collected and
subjected to
ammonium sulfate
precipitation
The pellet was
dissolved in
chromatography
buffer
The collected
elution with HGH
was concentrated
by ammonium
sulfate
precipitation
DEAE based
chromatograph
y was
performed
Final chromatography was done
with sepharyl CM and the elution
with HGH of 82% purity was
collected.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
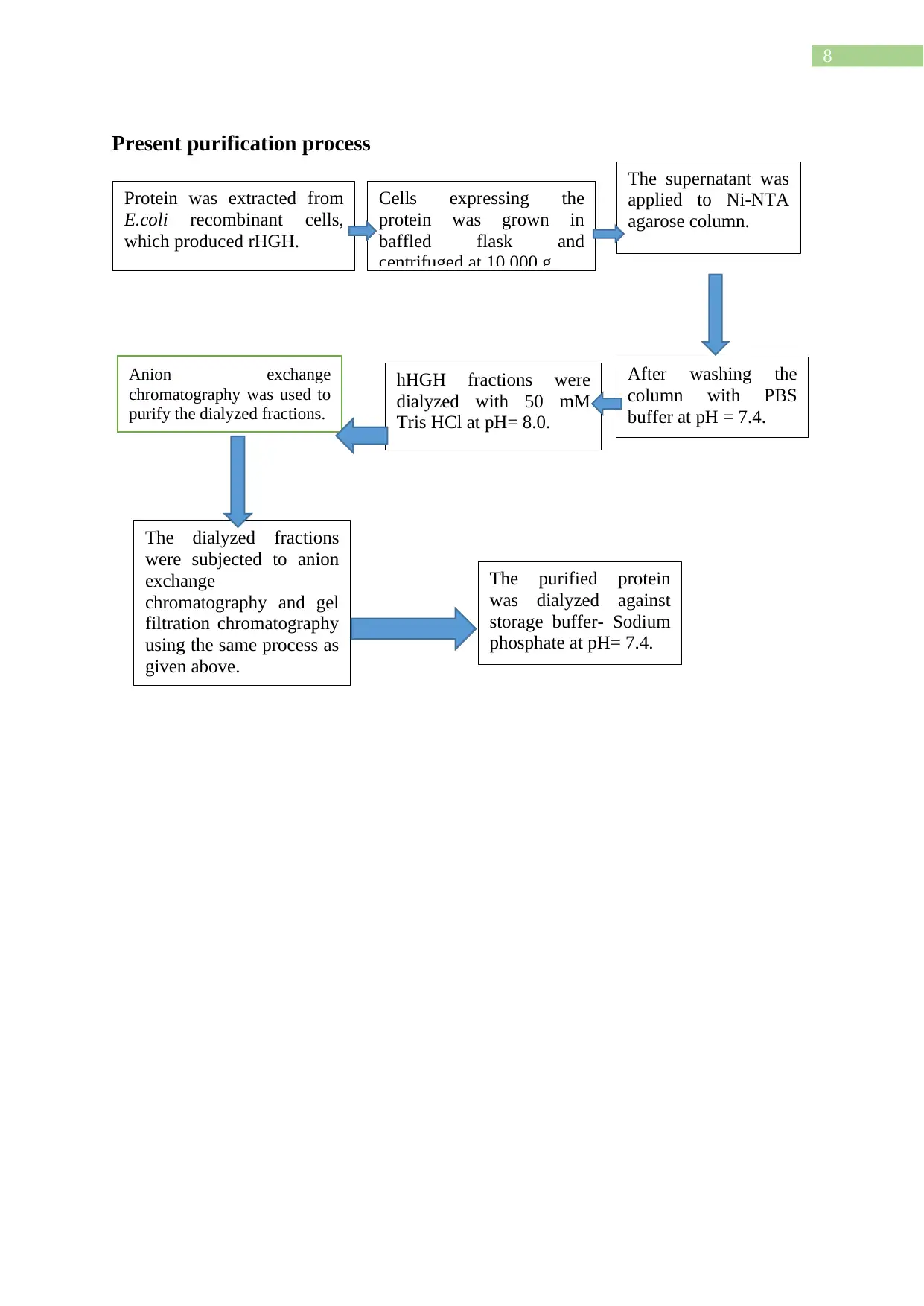
8
Present purification process
Protein was extracted from
E.coli recombinant cells,
which produced rHGH.
Cells expressing the
protein was grown in
baffled flask and
centrifuged at 10,000 g.
The supernatant was
applied to Ni-NTA
agarose column.
After washing the
column with PBS
buffer at pH = 7.4.
hHGH fractions were
dialyzed with 50 mM
Tris HCl at pH= 8.0.
Anion exchange
chromatography was used to
purify the dialyzed fractions.
The dialyzed fractions
were subjected to anion
exchange
chromatography and gel
filtration chromatography
using the same process as
given above.
The purified protein
was dialyzed against
storage buffer- Sodium
phosphate at pH= 7.4.
Present purification process
Protein was extracted from
E.coli recombinant cells,
which produced rHGH.
Cells expressing the
protein was grown in
baffled flask and
centrifuged at 10,000 g.
The supernatant was
applied to Ni-NTA
agarose column.
After washing the
column with PBS
buffer at pH = 7.4.
hHGH fractions were
dialyzed with 50 mM
Tris HCl at pH= 8.0.
Anion exchange
chromatography was used to
purify the dialyzed fractions.
The dialyzed fractions
were subjected to anion
exchange
chromatography and gel
filtration chromatography
using the same process as
given above.
The purified protein
was dialyzed against
storage buffer- Sodium
phosphate at pH= 7.4.

9
Bibliography
Aramvash, A., Sabet, A., Mansurpur, M., Azizi, A., Bahrami, A., & Kamali, N. (2018).
Comparison of Purification Processes for Recombinant Human Growth Hormone
Produced in E. coli. Iranian Journal of Science and Technology, Transactions A:
Science, 42(4), 1697-1705.
ExPASy
Kim, M. J., Park, H. S., Seo, K. H., Yang, H. J., Kim, S. K., & Choi, J. H. (2013). Complete
solubilization and purification of recombinant human growth hormone produced in
Escherichia coli. PLoS One, 8(2).
Levarski, Z., Šoltýsová, A., Krahulec, J., Stuchlík, S., & Turňa, J. (2014). High-level
expression and purification of recombinant human growth hormone produced in
soluble form in Escherichia coli. Protein expression and purification, 100, 40-47.
ProTaram
ProtScale
Puri, N. K., Crivelli, E., Cardamone, M., Fiddes, R., Bertolini, J., Ninham, B., & Brandon, M.
R. (1992). Solubilization of growth hormone and other recombinant proteins from
Escherichia coli inclusion bodies by using a cationic surfactant. Biochemical
Journal, 285(3), 871-879.
Song, H., Jiang, J., Wang, X., & Zhang, J. (2017). High purity recombinant human growth
hormone (rhGH) expression in Escherichia coli under phoA
promoter. Bioengineered, 8(2), 147-153.
Bibliography
Aramvash, A., Sabet, A., Mansurpur, M., Azizi, A., Bahrami, A., & Kamali, N. (2018).
Comparison of Purification Processes for Recombinant Human Growth Hormone
Produced in E. coli. Iranian Journal of Science and Technology, Transactions A:
Science, 42(4), 1697-1705.
ExPASy
Kim, M. J., Park, H. S., Seo, K. H., Yang, H. J., Kim, S. K., & Choi, J. H. (2013). Complete
solubilization and purification of recombinant human growth hormone produced in
Escherichia coli. PLoS One, 8(2).
Levarski, Z., Šoltýsová, A., Krahulec, J., Stuchlík, S., & Turňa, J. (2014). High-level
expression and purification of recombinant human growth hormone produced in
soluble form in Escherichia coli. Protein expression and purification, 100, 40-47.
ProTaram
ProtScale
Puri, N. K., Crivelli, E., Cardamone, M., Fiddes, R., Bertolini, J., Ninham, B., & Brandon, M.
R. (1992). Solubilization of growth hormone and other recombinant proteins from
Escherichia coli inclusion bodies by using a cationic surfactant. Biochemical
Journal, 285(3), 871-879.
Song, H., Jiang, J., Wang, X., & Zhang, J. (2017). High purity recombinant human growth
hormone (rhGH) expression in Escherichia coli under phoA
promoter. Bioengineered, 8(2), 147-153.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



