Qualitative Analysis of Ions: Chemistry Practical Report - Analysis
VerifiedAdded on 2022/09/08
|8
|1099
|9
Practical Assignment
AI Summary
This practical assignment provides a comprehensive overview of the qualitative analysis of various ions, including sodium, potassium, calcium, aluminum, copper, carbonate, sulfate, phosphate, chloride, and thiocyanate. The experiment involves observing characteristic reactions, such as flame tests, precipitate formation, and color changes upon the addition of specific reagents. The methodology includes preparing solutions, conducting flame tests, and adding specific reagents to test tubes containing the ions. The results section details the observed reactions for known and unknown solutions, and the discussion section analyzes the findings. The conclusion highlights the identified ions in the unknown solution. The report also includes a list of references from chemistry textbooks and journals.

1
QUALITATIVE ANALYSIS OF IONS
Name:
School:
Department:
Date:
QUALITATIVE ANALYSIS OF IONS
Name:
School:
Department:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
Introduction
An ion is denoted as atom groups or atom that possesses a net charge. An atom hold a net
positive charge if it loses one or more electrons. Monatomic ions can be defined as ions created
from single atoms whereas a polyatomic ion is a class of atoms with a fixed composition,
attached together by a covalent link which has a net charge. In analytical chemistry field,
chemical properties of sample components are identified qualitatively or quantitatively1.
The analysis of ions qualitatively depends on checking the ions reactions characteristics. Several
ions have distinct chemical properties that can be utilized in their identification, though some
have same chemical properties and will tend to interfere with one another when existing in the
mixtures. It is due to their ability to emit light of characteristics odors when exposed to flames,
where there are changed to gaseous metal atoms. A good example is whereby sodium emits
yellow light color noted in a sodium street lamp2.
Materials
1 Bell, Colin Frank. Syntheses and physical studies of inorganic compounds. Elsevier,
2013. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=8jcXBQAAQBAJ&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=X
0sScqj4yQ&sig=hjRpb1FOfHVBgBqdCRLyWYtPohA
2 Banu, K. Sahira, and L. Cathrine. "General techniques involved in phytochemical
aanlysis." International Journal of Advanced Research in Chemical Science 2, no. 4
(2015): 25-32. [Online]. Available fro m:
https://www.academia.edu/download/56221117/5.pdf
Introduction
An ion is denoted as atom groups or atom that possesses a net charge. An atom hold a net
positive charge if it loses one or more electrons. Monatomic ions can be defined as ions created
from single atoms whereas a polyatomic ion is a class of atoms with a fixed composition,
attached together by a covalent link which has a net charge. In analytical chemistry field,
chemical properties of sample components are identified qualitatively or quantitatively1.
The analysis of ions qualitatively depends on checking the ions reactions characteristics. Several
ions have distinct chemical properties that can be utilized in their identification, though some
have same chemical properties and will tend to interfere with one another when existing in the
mixtures. It is due to their ability to emit light of characteristics odors when exposed to flames,
where there are changed to gaseous metal atoms. A good example is whereby sodium emits
yellow light color noted in a sodium street lamp2.
Materials
1 Bell, Colin Frank. Syntheses and physical studies of inorganic compounds. Elsevier,
2013. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=8jcXBQAAQBAJ&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=X
0sScqj4yQ&sig=hjRpb1FOfHVBgBqdCRLyWYtPohA
2 Banu, K. Sahira, and L. Cathrine. "General techniques involved in phytochemical
aanlysis." International Journal of Advanced Research in Chemical Science 2, no. 4
(2015): 25-32. [Online]. Available fro m:
https://www.academia.edu/download/56221117/5.pdf

3
Chemicals
0.2MNACL, 6MHCL, 1M Na2C2O4, 0.2MKCL, 0.1MCa(NO3)2, 0.5MAlCl3, Al(OH)3,
NH4OH, 3M HC2H302, 1M Na2CO3, 1m HCl, 1M Na2CO3, 0.5M NaSO4, NaOH,
AgN03, distilled water, AgNO3, 05M KSCN, 0.1MFe (NO3)3, 05M (NH4)2 MoO4, 05M
CuSO4, 1M BaCl2, 6MHNO3, 01M Na3PO4
Equipment
Test tubes, flame test wire, stirring rods, Bunsen burner, beaker, hot water bath, test tube clump,
well plate and utility clamp
Methods
Sodium ions
The flame test wire is cleaned first while dipping it in HCL, and then heated on the hottest part of
flame. Then, then the test wire is dipped in 0.2M of NaCl and placed in the flame. A yellow
flame indicates a positive test for the sodium ions3.
Potassium ions
The flame test wire is dipped in the test tube containing 0.2MKCl and placed in the flame. A
purple to orange color shows the presence of potassium ions.
3 Perry, Dale L. Handbook of inorganic compounds. CRC press, 2016. [Online]. Available
from: https://books.google.com/books?
hl=en&lr=&id=SFD30BvPBhoC&oi=fnd&pg=PP1&dq=many+of+
+inorganic+compounds+forms+white+precipatate+when+
+heated&ots=uqOpiExSMw&sig=svi-Uaidm0CPIusyPI8Me_lIBCE
Chemicals
0.2MNACL, 6MHCL, 1M Na2C2O4, 0.2MKCL, 0.1MCa(NO3)2, 0.5MAlCl3, Al(OH)3,
NH4OH, 3M HC2H302, 1M Na2CO3, 1m HCl, 1M Na2CO3, 0.5M NaSO4, NaOH,
AgN03, distilled water, AgNO3, 05M KSCN, 0.1MFe (NO3)3, 05M (NH4)2 MoO4, 05M
CuSO4, 1M BaCl2, 6MHNO3, 01M Na3PO4
Equipment
Test tubes, flame test wire, stirring rods, Bunsen burner, beaker, hot water bath, test tube clump,
well plate and utility clamp
Methods
Sodium ions
The flame test wire is cleaned first while dipping it in HCL, and then heated on the hottest part of
flame. Then, then the test wire is dipped in 0.2M of NaCl and placed in the flame. A yellow
flame indicates a positive test for the sodium ions3.
Potassium ions
The flame test wire is dipped in the test tube containing 0.2MKCl and placed in the flame. A
purple to orange color shows the presence of potassium ions.
3 Perry, Dale L. Handbook of inorganic compounds. CRC press, 2016. [Online]. Available
from: https://books.google.com/books?
hl=en&lr=&id=SFD30BvPBhoC&oi=fnd&pg=PP1&dq=many+of+
+inorganic+compounds+forms+white+precipatate+when+
+heated&ots=uqOpiExSMw&sig=svi-Uaidm0CPIusyPI8Me_lIBCE
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
Calcium ions
2-3 drops of 1M sodium oxalate is added to a test tube containing calcium nitrate. Appearance of
white precipitate confirms the calcium ions presence. In another test tube, 3 drops of 6M HCl is
added to 0.1M calcium nitrates and flame test is performed. If the orange-red flame is visible
then there is presence of calcium ions.
Aluminum ions
10 drops of aluminum fluoride is added drop wise to a test tube having ammonium hydroxides.
A white precipitate of Al (OH) 3 is formed. Once again, drop wise of acetic acid is added drop
wise until the solid dissolves. Two drops of Catechol violet reagent complex is added to forms a
blue suspension.
Copper ions
To a test having 10 drops of copper sulphate, ammonia hydroxide is added drop by drop. A blue
complex solution will develops.
Carbonates
10 drops of 6MHCl is added to 10 drops of 1M NaCO3 in a test tube. The appearance of odorless
and colorless gas with CO2 bubbles indicates the presence of carbonates.
Sulfates
10 drops of 1MHCl is added carefully to a test tube having 10 drops of 0.5M Na2SO4 and mixed
properly. 2-3 drops of 1M BaCl2 is added. The presence of sulfate ions is indicated by
appearance of white powdery precipitate.
Calcium ions
2-3 drops of 1M sodium oxalate is added to a test tube containing calcium nitrate. Appearance of
white precipitate confirms the calcium ions presence. In another test tube, 3 drops of 6M HCl is
added to 0.1M calcium nitrates and flame test is performed. If the orange-red flame is visible
then there is presence of calcium ions.
Aluminum ions
10 drops of aluminum fluoride is added drop wise to a test tube having ammonium hydroxides.
A white precipitate of Al (OH) 3 is formed. Once again, drop wise of acetic acid is added drop
wise until the solid dissolves. Two drops of Catechol violet reagent complex is added to forms a
blue suspension.
Copper ions
To a test having 10 drops of copper sulphate, ammonia hydroxide is added drop by drop. A blue
complex solution will develops.
Carbonates
10 drops of 6MHCl is added to 10 drops of 1M NaCO3 in a test tube. The appearance of odorless
and colorless gas with CO2 bubbles indicates the presence of carbonates.
Sulfates
10 drops of 1MHCl is added carefully to a test tube having 10 drops of 0.5M Na2SO4 and mixed
properly. 2-3 drops of 1M BaCl2 is added. The presence of sulfate ions is indicated by
appearance of white powdery precipitate.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
Phosphate ions
10 drops of 6MHNO3 is added to test tube having 10 drops of 0.1M Na3PO4 and then 10 drops
of 0.5 M(NH4)2MoO4 is added and mixed. The test tube is heated in boiling water bath for
approximately 5 minutes. The test tube is taken out and allowed to stand for 10 minutes. Fine
pale yellow presence indicates presence of phosphate ions.
Chloride ions
Into 10 drop test tube having 0.2M NaCl, 5 drops of 1M HNO3 is added. 2-3 drops of 0.1M
AgNO3 is added. A white precipitate of AgCl denotes the presence of Cl- ions.
Thiocyanate ions
To a test tube containing 10 drops of 0.5 MKSCN, 10 drops of HC2H3O2 is added and mixed.
0.5 M Fe (NO3)3 is added drop wise until a blood red color appears which shows the presence of
SCN- ion.
Results
Ion tested Known Unknown
Na+ Yellow flame +
K+ Orange-yellow +
Ca2+ Orange-red flame -
Al3+ On additional of ammonium
hydroxide, white precipitate
was formed. Blue suspension
-
Phosphate ions
10 drops of 6MHNO3 is added to test tube having 10 drops of 0.1M Na3PO4 and then 10 drops
of 0.5 M(NH4)2MoO4 is added and mixed. The test tube is heated in boiling water bath for
approximately 5 minutes. The test tube is taken out and allowed to stand for 10 minutes. Fine
pale yellow presence indicates presence of phosphate ions.
Chloride ions
Into 10 drop test tube having 0.2M NaCl, 5 drops of 1M HNO3 is added. 2-3 drops of 0.1M
AgNO3 is added. A white precipitate of AgCl denotes the presence of Cl- ions.
Thiocyanate ions
To a test tube containing 10 drops of 0.5 MKSCN, 10 drops of HC2H3O2 is added and mixed.
0.5 M Fe (NO3)3 is added drop wise until a blood red color appears which shows the presence of
SCN- ion.
Results
Ion tested Known Unknown
Na+ Yellow flame +
K+ Orange-yellow +
Ca2+ Orange-red flame -
Al3+ On additional of ammonium
hydroxide, white precipitate
was formed. Blue suspension
-
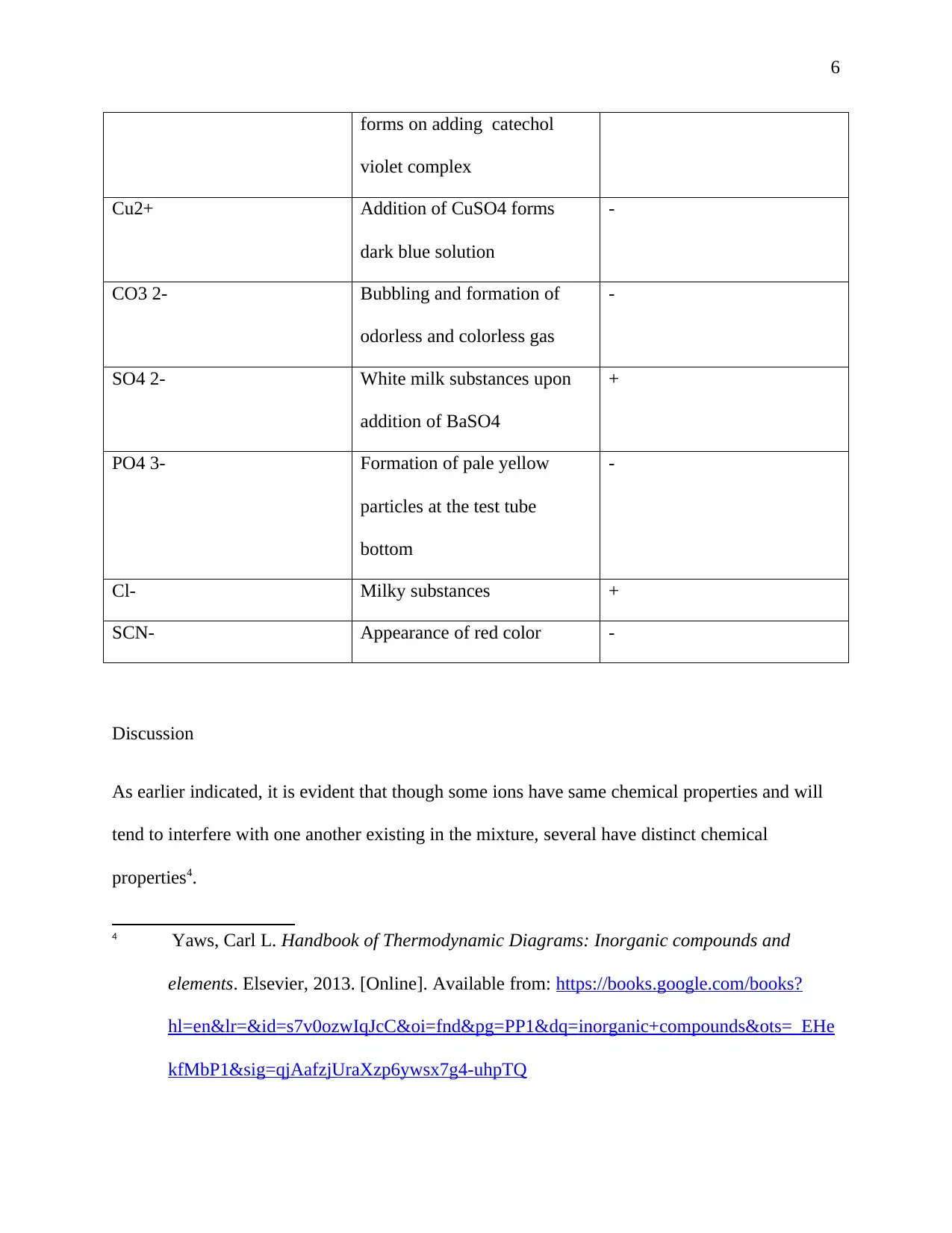
6
forms on adding catechol
violet complex
Cu2+ Addition of CuSO4 forms
dark blue solution
-
CO3 2- Bubbling and formation of
odorless and colorless gas
-
SO4 2- White milk substances upon
addition of BaSO4
+
PO4 3- Formation of pale yellow
particles at the test tube
bottom
-
Cl- Milky substances +
SCN- Appearance of red color -
Discussion
As earlier indicated, it is evident that though some ions have same chemical properties and will
tend to interfere with one another existing in the mixture, several have distinct chemical
properties4.
4 Yaws, Carl L. Handbook of Thermodynamic Diagrams: Inorganic compounds and
elements. Elsevier, 2013. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=s7v0ozwIqJcC&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=_EHe
kfMbP1&sig=qjAafzjUraXzp6ywsx7g4-uhpTQ
forms on adding catechol
violet complex
Cu2+ Addition of CuSO4 forms
dark blue solution
-
CO3 2- Bubbling and formation of
odorless and colorless gas
-
SO4 2- White milk substances upon
addition of BaSO4
+
PO4 3- Formation of pale yellow
particles at the test tube
bottom
-
Cl- Milky substances +
SCN- Appearance of red color -
Discussion
As earlier indicated, it is evident that though some ions have same chemical properties and will
tend to interfere with one another existing in the mixture, several have distinct chemical
properties4.
4 Yaws, Carl L. Handbook of Thermodynamic Diagrams: Inorganic compounds and
elements. Elsevier, 2013. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=s7v0ozwIqJcC&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=_EHe
kfMbP1&sig=qjAafzjUraXzp6ywsx7g4-uhpTQ
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
Conclusion
It can be concluded that in the solution of unknown, Na+, K+, SO4 2-, and Cl- were present in
the solution.
Conclusion
It can be concluded that in the solution of unknown, Na+, K+, SO4 2-, and Cl- were present in
the solution.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
Bibliography
Banu, K. Sahira, and L. Cathrine. "General techniques involved in phytochemical
aanlysis." International Journal of Advanced Research in Chemical Science 2, no. 4
(2015): 25-32. [Online]. Available fro m:
https://www.academia.edu/download/56221117/5.pdf
Bell, Colin Frank. Syntheses and physical studies of inorganic compounds. Elsevier, 2013.
[Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=8jcXBQAAQBAJ&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=X
0sScqj4yQ&sig=hjRpb1FOfHVBgBqdCRLyWYtPohA
Perry, Dale L. Handbook of inorganic compounds. CRC press, 2016. [Online]. Available from:
https://books.google.com/books?
hl=en&lr=&id=SFD30BvPBhoC&oi=fnd&pg=PP1&dq=many+of+
+inorganic+compounds+forms+white+precipatate+when+
+heated&ots=uqOpiExSMw&sig=svi-Uaidm0CPIusyPI8Me_lIBCE
Yaws, Carl L. Handbook of Thermodynamic Diagrams: Inorganic compounds and elements.
Elsevier, 2013. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=s7v0ozwIqJcC&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=_EH
ekfMbP1&sig=qjAafzjUraXzp6ywsx7g4-uhpTQ
Bibliography
Banu, K. Sahira, and L. Cathrine. "General techniques involved in phytochemical
aanlysis." International Journal of Advanced Research in Chemical Science 2, no. 4
(2015): 25-32. [Online]. Available fro m:
https://www.academia.edu/download/56221117/5.pdf
Bell, Colin Frank. Syntheses and physical studies of inorganic compounds. Elsevier, 2013.
[Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=8jcXBQAAQBAJ&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=X
0sScqj4yQ&sig=hjRpb1FOfHVBgBqdCRLyWYtPohA
Perry, Dale L. Handbook of inorganic compounds. CRC press, 2016. [Online]. Available from:
https://books.google.com/books?
hl=en&lr=&id=SFD30BvPBhoC&oi=fnd&pg=PP1&dq=many+of+
+inorganic+compounds+forms+white+precipatate+when+
+heated&ots=uqOpiExSMw&sig=svi-Uaidm0CPIusyPI8Me_lIBCE
Yaws, Carl L. Handbook of Thermodynamic Diagrams: Inorganic compounds and elements.
Elsevier, 2013. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=s7v0ozwIqJcC&oi=fnd&pg=PP1&dq=inorganic+compounds&ots=_EH
ekfMbP1&sig=qjAafzjUraXzp6ywsx7g4-uhpTQ
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


