Year 12 Physics: Radioactivity and Its Applications Report
VerifiedAdded on 2022/11/28
|10
|1683
|412
Report
AI Summary
This report provides a comprehensive overview of radioactivity and its applications. It begins with a definition of radioactivity as the spontaneous emission of particles from an unstable nuclide, and then details the three main types of radiation: alpha, beta, and gamma. For each type, the report discusses its properties, safety measures to be taken when handling it, and various applications. Alpha radiation is described as positively charged helium nuclei used in smoke detectors and static eliminators, while beta radiation, negatively charged electrons, finds use in thickness gauges and cancer treatment. Gamma radiation, high-energy photons, is applied in medical treatments and industrial processes. The report includes a discussion of nuclear reactions and relevant references.

Radioactivity 1
Radioactivity and its application
By (name of the student)
Class name
(Course name)
Professor (Tutor)
The Name of the School (University)
The City and State where it is located
The Date
Radioactivity and its application
By (name of the student)
Class name
(Course name)
Professor (Tutor)
The Name of the School (University)
The City and State where it is located
The Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Radioactivity 2
Radioactivity and its application
According to Poenaru and Iva (2016) “radioactivity is a spontaneous random emission of
particles from the nucleus of an unstable nuclide.” This process is not dependent on the external
factors such as temperature, pressure or chemical composition.
In a radioactive process, radiations are emitted from the nucleus of an atom. The popular
forms of radiations are alpha (a), beta (B) and gamma (g). These radiations originating from
nucleus are referred to as nuclear reactions (Goldstein and Wilkins, 2011). These radiations are
identified as per the characteristics the exhibit.
According to Crundell, M. (2014) “the nuclides can either be stable or unstable, the stable
nuclides have a neutron/proton (n/p) ratio of around 1:1.” An example is calcium (Ca) with 20
neutrons and 20 protons, thus having the n/p ratio of 20:20= 1:1. Nuclides with a higher atomic
numbers have n/p ratio increasing gradually to about 1.6:1 which is still in the stability section.
Above this ratio the nucleus becomes huge and unstable, hence chances of undergoing
radioactive decay rise.
The following are the three forms of radiation forms of radiations.
Alpha radiation
Alpha emissions are positively charged helium nuclei, represented as 2
4
He2+ in a nuclear
reactions. If a nuclide decays by liberating an alpha particle, the mass number reduces by 4 and
the proton number by 2.
Radioactivity and its application
According to Poenaru and Iva (2016) “radioactivity is a spontaneous random emission of
particles from the nucleus of an unstable nuclide.” This process is not dependent on the external
factors such as temperature, pressure or chemical composition.
In a radioactive process, radiations are emitted from the nucleus of an atom. The popular
forms of radiations are alpha (a), beta (B) and gamma (g). These radiations originating from
nucleus are referred to as nuclear reactions (Goldstein and Wilkins, 2011). These radiations are
identified as per the characteristics the exhibit.
According to Crundell, M. (2014) “the nuclides can either be stable or unstable, the stable
nuclides have a neutron/proton (n/p) ratio of around 1:1.” An example is calcium (Ca) with 20
neutrons and 20 protons, thus having the n/p ratio of 20:20= 1:1. Nuclides with a higher atomic
numbers have n/p ratio increasing gradually to about 1.6:1 which is still in the stability section.
Above this ratio the nucleus becomes huge and unstable, hence chances of undergoing
radioactive decay rise.
The following are the three forms of radiation forms of radiations.
Alpha radiation
Alpha emissions are positively charged helium nuclei, represented as 2
4
He2+ in a nuclear
reactions. If a nuclide decays by liberating an alpha particle, the mass number reduces by 4 and
the proton number by 2.
Radioactivity 3
Properties of alpha radiation
As argued by Lowenthal and Airey (2001), alpha particles have possess an electric charge
of 2+. They are therefore attracted to negative plate in an electric field. They are also less
deflected in the electric field as compared to a beta particles due to its heaviness.
Alpha particles have a very high ionizing power due to electric charge of 2+ its particles
have. Large number of ions are therefore produced as they pass through due to their charge and
slow speeds. Slow speeds gives time the alpha particles to be in contact with the gasses for a
relatively longer time.
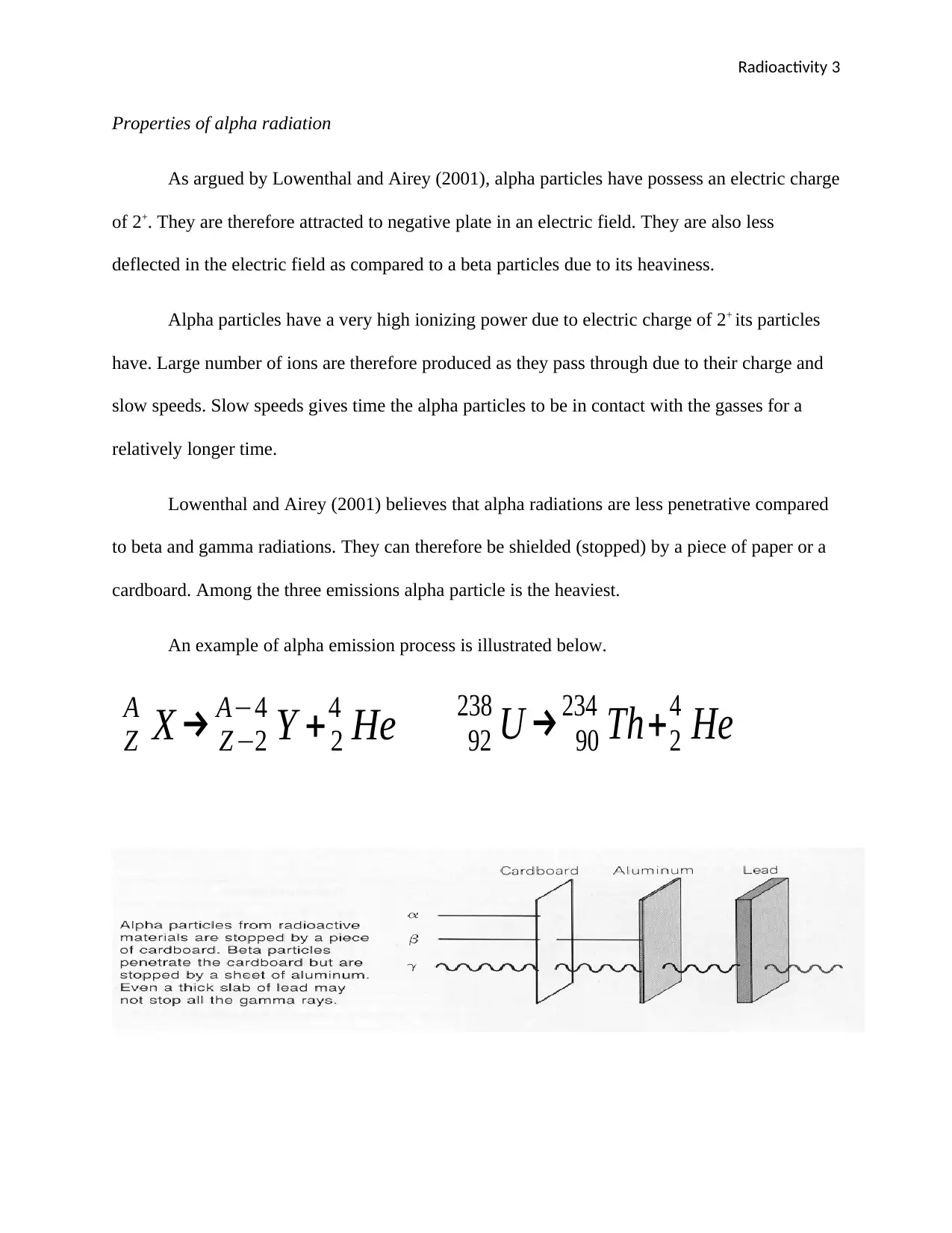
Lowenthal and Airey (2001) believes that alpha radiations are less penetrative compared
to beta and gamma radiations. They can therefore be shielded (stopped) by a piece of paper or a
cardboard. Among the three emissions alpha particle is the heaviest.
An example of alpha emission process is illustrated below.
Z
A X → Z−2
A−4 Y + 2
4 He 92
238 U → 90
234 Th+2
4 He
Properties of alpha radiation
As argued by Lowenthal and Airey (2001), alpha particles have possess an electric charge
of 2+. They are therefore attracted to negative plate in an electric field. They are also less
deflected in the electric field as compared to a beta particles due to its heaviness.
Alpha particles have a very high ionizing power due to electric charge of 2+ its particles
have. Large number of ions are therefore produced as they pass through due to their charge and
slow speeds. Slow speeds gives time the alpha particles to be in contact with the gasses for a
relatively longer time.
Lowenthal and Airey (2001) believes that alpha radiations are less penetrative compared
to beta and gamma radiations. They can therefore be shielded (stopped) by a piece of paper or a
cardboard. Among the three emissions alpha particle is the heaviest.
An example of alpha emission process is illustrated below.
Z
A X → Z−2
A−4 Y + 2
4 He 92
238 U → 90
234 Th+2
4 He
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Radioactivity 4
Safety measures needed when handling alpha radiation.
Alpha particles are more dangerous if it enters the open wound, ingested (swallowed) or
breathed in.
Precautions.
Limiting exposure time and keeping the radioactive sources shielded with led lined box
minimizes damage to body health functioning.
Putting on protective clothing to prevent the body coming in contact with the radiations is
the best way.
Using detector badges to monitor the exposure and handling radioactive elements using
equipment such as thongs to increase the distance from the source.
Uses of alpha radiations
In Jityaev et al. (2017) “alpha radiations can be used in smoke detectors.” 241- Americium
is used to ionize smoke detectors. If by chance the smoke enters the detector, the alpha particles
in it are reduced therefore activating the alarm.
Used in static eliminators to discharge static charges from equipment this alpha particles
are obtained from 210-Polonium.
According to Kitis and Charalambus (2009), thermoelectric generators uses alpha
particles/radiations from decaying 238-Plutonium to generate heat which is transformed to
electricity.
Safety measures needed when handling alpha radiation.
Alpha particles are more dangerous if it enters the open wound, ingested (swallowed) or
breathed in.
Precautions.
Limiting exposure time and keeping the radioactive sources shielded with led lined box
minimizes damage to body health functioning.
Putting on protective clothing to prevent the body coming in contact with the radiations is
the best way.
Using detector badges to monitor the exposure and handling radioactive elements using
equipment such as thongs to increase the distance from the source.
Uses of alpha radiations
In Jityaev et al. (2017) “alpha radiations can be used in smoke detectors.” 241- Americium
is used to ionize smoke detectors. If by chance the smoke enters the detector, the alpha particles
in it are reduced therefore activating the alarm.
Used in static eliminators to discharge static charges from equipment this alpha particles
are obtained from 210-Polonium.
According to Kitis and Charalambus (2009), thermoelectric generators uses alpha
particles/radiations from decaying 238-Plutonium to generate heat which is transformed to
electricity.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Radioactivity 5
Beta radiations
Beta radiations are particles which are negatively charged, denoted as
−1
0
b. Therefore
they electrons originating from inside the nucleus and not from the outer energy levels. Beta
particles are formed when neutron changes into a proton inside the nucleus.
Beta emission is illustrated in the figure below
Z
A X →Z +1
A Y + e−
6
14 Cu→ 7
14 Ni+ e−
64-copper undergoes radioactive decay by emitting 64-nickel and a beta particle to
become stable.
Properties of gamma radiations.
They lose energy very fast as they interact with matter due to their light mass, and have a
random path too as a result of that.
They can attain the speed of light when accelerated due to small mass. They ionize less
than alpha particle hence are less lethal than alpha particles for given amount of energy
deposition.
Beta radiations
Beta radiations are particles which are negatively charged, denoted as
−1
0
b. Therefore
they electrons originating from inside the nucleus and not from the outer energy levels. Beta
particles are formed when neutron changes into a proton inside the nucleus.
Beta emission is illustrated in the figure below
Z
A X →Z +1
A Y + e−
6
14 Cu→ 7
14 Ni+ e−
64-copper undergoes radioactive decay by emitting 64-nickel and a beta particle to
become stable.
Properties of gamma radiations.
They lose energy very fast as they interact with matter due to their light mass, and have a
random path too as a result of that.
They can attain the speed of light when accelerated due to small mass. They ionize less
than alpha particle hence are less lethal than alpha particles for given amount of energy
deposition.

Radioactivity 6
They are negatively charged hence get attracted to the positively plate of an electric field
and carry a single negative charge (-1).
Safety measures needed when handling beta radiation
According to Johns (1987), though beta particles are less ionizing than alpha particles,
beta particles have a higher penetrating power. It can therefore go through many millimeters of
skin or tissue. They can cause burns like severe sunburn if exposed to high intensity beta
radiation. Toxic too when ingested or inhaled and can damage organs and tissues extensively.
Precautions
Minimizing time of exposure- radiation amount is directly proportional to the duration of
exposure to the radiation particles.
Stay time= Exposure quantity
Increasing the distance from the source of radiation- doubling the distance minimizes the number
of beta particles reaching where you are ¼ the initial exposure rate. And reducing the distance by 2
doubles the exposure by four.
And shielding the body from coming into contact with the radiation- a barrier also called shield
reduces the intensity of radiation significantly by absorbing or stopping the radiation from reaching
unintended objects or bodies, Johns (1987). Beta can be shielded by wearing specialized heavy clothing
or a thin metallic foil.
Uses of beta radiation
They are negatively charged hence get attracted to the positively plate of an electric field
and carry a single negative charge (-1).
Safety measures needed when handling beta radiation
According to Johns (1987), though beta particles are less ionizing than alpha particles,
beta particles have a higher penetrating power. It can therefore go through many millimeters of
skin or tissue. They can cause burns like severe sunburn if exposed to high intensity beta
radiation. Toxic too when ingested or inhaled and can damage organs and tissues extensively.
Precautions
Minimizing time of exposure- radiation amount is directly proportional to the duration of
exposure to the radiation particles.
Stay time= Exposure quantity
Increasing the distance from the source of radiation- doubling the distance minimizes the number
of beta particles reaching where you are ¼ the initial exposure rate. And reducing the distance by 2
doubles the exposure by four.
And shielding the body from coming into contact with the radiation- a barrier also called shield
reduces the intensity of radiation significantly by absorbing or stopping the radiation from reaching
unintended objects or bodies, Johns (1987). Beta can be shielded by wearing specialized heavy clothing
or a thin metallic foil.
Uses of beta radiation
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Radioactivity 7
The significant penetration of these type of radiation has beneficial applications.
Can be used on quality assurance to check on thickness of thin materials such as papers
and clothing.
90-strontium and 89-strontium are used in treatment of eye and bone cancers.
Gamma radiations
According to Goldstein et al. (2011) “gamma radiations are high energy photons that are
emitted by radioactive decaying nuclide and are very high energy form of ionizing radiation with
a much shorter wavelength and very high frequency.”
Gamma radiation is produced as a form of energy by unstable excited nuclide so as to
achieve stability.
Example is cobalt-60 which might be in excited state and in order to attain stability it
emits gamma rays without producing new isotope.
60
27 Co
60
27 Co + y (gamma rays)
Properties
The significant penetration of these type of radiation has beneficial applications.
Can be used on quality assurance to check on thickness of thin materials such as papers
and clothing.
90-strontium and 89-strontium are used in treatment of eye and bone cancers.
Gamma radiations
According to Goldstein et al. (2011) “gamma radiations are high energy photons that are
emitted by radioactive decaying nuclide and are very high energy form of ionizing radiation with
a much shorter wavelength and very high frequency.”
Gamma radiation is produced as a form of energy by unstable excited nuclide so as to
achieve stability.
Example is cobalt-60 which might be in excited state and in order to attain stability it
emits gamma rays without producing new isotope.
60
27 Co
60
27 Co + y (gamma rays)
Properties
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Radioactivity 8
They do not have an electric charge, hence cannot be affected by magnetic field in any
way.
They are not emitted on their own, they are emitted with one of the two the named
radiations above or both.
Gruzin and Babikova (1961, p.750-752) argue that gamma rays has highest penetrating
power and can go through sheet of paper and aluminum foil and can only be stopped by thick
lead block.
Safety measures needed when handling the gamma radiations.
Due to its high penetrating power and high energy radiation sources must be shielded
with 1.3 feet thick lead metal.
Uses of gamma radiations
Medical treatment- its ability to ionize tissue and cause cancer has been applied to kill
bacteria and malignant cells.
In industries- used to determine metal sheets thickness and detecting flaws in the metals by
metals making industries.
According to Joseph and Peterson (2003), gamma radiation can be used in food industry to
sterilize food by killing the microorganisms before storing them to prevent decomposition of the food.
They do not have an electric charge, hence cannot be affected by magnetic field in any
way.
They are not emitted on their own, they are emitted with one of the two the named
radiations above or both.
Gruzin and Babikova (1961, p.750-752) argue that gamma rays has highest penetrating
power and can go through sheet of paper and aluminum foil and can only be stopped by thick
lead block.
Safety measures needed when handling the gamma radiations.
Due to its high penetrating power and high energy radiation sources must be shielded
with 1.3 feet thick lead metal.
Uses of gamma radiations
Medical treatment- its ability to ionize tissue and cause cancer has been applied to kill
bacteria and malignant cells.
In industries- used to determine metal sheets thickness and detecting flaws in the metals by
metals making industries.
According to Joseph and Peterson (2003), gamma radiation can be used in food industry to
sterilize food by killing the microorganisms before storing them to prevent decomposition of the food.

Radioactivity 9
References
Chemed.chem.purdue.edu. (2019). Radioactive Decay. [online] Available at:
https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch23/modes.php [Accessed 26 Jun.
2019].
Crundell, M. (2014). Cambridge International AS and A Level Physics 2nd ed. London: Hodder Education.
Goldstein, H. and Wilkins, J. (2011). Calculations of the penetration of gamma rays. Oak Ridge,
Tennessee: United States Atomic Energy Commission.
Gruzin, P. and Babikova, Y. (2012). Uses of radioactive isotopes and nuclear radiations in metallurgy. The
Soviet Journal of Atomic Energy, New York: Consultant Bureau Enterprises.
Jityaev, I., Svetlichnyi, A., Kolomiytsev, A. and Ageev, O. (2017). Screening effect in matrix grapheme / Sic
planar field emitters. Journal of Physics, Bristol, United Kingdom: Institute of physics.
Johanna, L. Miller (2013). Energetic gamma rays on Earth, Physics Today. College Park, Maryland:
American institute of physics.
Johns T.F. (1987). Radiation Protection Dosimetry, volume 9. Oxford, United Kingdom: oxford university
press.
Josephson, E. and Peterson, M. (2003). Preservation of food by ionizing radiation. 4th ed. Sawston
Cambridge: Woodhead publishing limited.
References
Chemed.chem.purdue.edu. (2019). Radioactive Decay. [online] Available at:
https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch23/modes.php [Accessed 26 Jun.
2019].
Crundell, M. (2014). Cambridge International AS and A Level Physics 2nd ed. London: Hodder Education.
Goldstein, H. and Wilkins, J. (2011). Calculations of the penetration of gamma rays. Oak Ridge,
Tennessee: United States Atomic Energy Commission.
Gruzin, P. and Babikova, Y. (2012). Uses of radioactive isotopes and nuclear radiations in metallurgy. The
Soviet Journal of Atomic Energy, New York: Consultant Bureau Enterprises.
Jityaev, I., Svetlichnyi, A., Kolomiytsev, A. and Ageev, O. (2017). Screening effect in matrix grapheme / Sic
planar field emitters. Journal of Physics, Bristol, United Kingdom: Institute of physics.
Johanna, L. Miller (2013). Energetic gamma rays on Earth, Physics Today. College Park, Maryland:
American institute of physics.
Johns T.F. (1987). Radiation Protection Dosimetry, volume 9. Oxford, United Kingdom: oxford university
press.
Josephson, E. and Peterson, M. (2003). Preservation of food by ionizing radiation. 4th ed. Sawston
Cambridge: Woodhead publishing limited.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Radioactivity 10
Kitis, G. and Charalambous, S. (2009). Relative thermoluminescence response of alpha to beta radiation
as a function of irradiation temperature. Amsterdam Netherlands: Elsevier Science Publishers B.V.
Lowenthal, G. and Airey, P. (2001). Practical Applications of Radioactivity and Nuclear Radiations.
Cambridge: Cambridge University Press.
Mishra, A. (2012). Transition metals. New York: Nova Science Publishers.
Poenaru, D. and Iva, M. (1989). Particle Emission from Nuclei. 1st ed. Boca Raton, Florida: CRC Press.
Kitis, G. and Charalambous, S. (2009). Relative thermoluminescence response of alpha to beta radiation
as a function of irradiation temperature. Amsterdam Netherlands: Elsevier Science Publishers B.V.
Lowenthal, G. and Airey, P. (2001). Practical Applications of Radioactivity and Nuclear Radiations.
Cambridge: Cambridge University Press.
Mishra, A. (2012). Transition metals. New York: Nova Science Publishers.
Poenaru, D. and Iva, M. (1989). Particle Emission from Nuclei. 1st ed. Boca Raton, Florida: CRC Press.
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





