Analysis of Corrosion and Deterioration in RC Bridge Structures
VerifiedAdded on 2022/11/13
|29
|7341
|289
Project
AI Summary
This project investigates the corrosion and deterioration of reinforced concrete (RC) structures, specifically focusing on a bridge girder. The study examines the causes of concrete deterioration, with a primary focus on the corrosion of steel reinforcement. The project explores the mechanisms of corrosion, including electrochemical processes, pitting corrosion, and the influence of environmental factors such as moisture, oxygen, carbonation, aggressive anions (chloride), and bacteria. The research delves into the effects of corrosion on structural soundness, including concrete cracking, spalling, and a reduction in load-bearing capacity. Various tests, including non-destructive testing and visual inspection, are employed to assess the extent of corrosion. The project also discusses the internal and external factors affecting corrosion, such as cement composition and environmental conditions. The document provides detailed analysis of corrosion processes, factors influencing corrosion rates, and the impact on the durability and service life of RC structures.

Corrosion and Deterioration of RC structure 1
CORROSION AND DETERIORATION OF RC STRUCTURE
Name
Course
Professor
University
City/state
Date
CORROSION AND DETERIORATION OF RC STRUCTURE
Name
Course
Professor
University
City/state
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Corrosion and Deterioration of RC structure 2
Corrosion and Deterioration of RC Structure
Summary
Structural concrete can deteriorate due to several factors including physical and chemical effects.
One of the major causes of concrete structures deterioration today is corrosion of reinforced
steel. Occurrence of corrosion results to structural weakening, which is caused by a decrease in
cross-section of the steel, concrete spalling, concrete cracking, surface staining, and concrete
delamination. The corrosion ends up reducing the reinforced concrete (RC) structure’s service
life. The main problem associated with the safety and structural soundness or integrity of RC
structures is the reduction of its load-bearing capacity. The main purpose of this project was to
investigate the extent of corrosion and deterioration of RC structure – a bridge girder, and to
establish potential factors that contributes to deterioration of bridges in general. This was
attained by carrying out several tests and investigations including non-destructive testing and
visual inspection of the bridge.
Corrosion of Steel Reinforcement in RC Structures
Corrosion of reinforcement has a significant effect on the durability of RC structures hence it is
worth being investigated. The damage of concrete caused by corrosion is among the leading
causes of decreased durability of RC structures. A study conducted by researchers worldwide
found that steel reinforcement rusting causes more than four-hundredth of structural failures
across the world.
Effect of Reinforcement Corrosion on Structural Soundness
There are two major damaging effects associated with corrosion of steel reinforcement of
concrete structures. These are:
Corrosion and Deterioration of RC Structure
Summary
Structural concrete can deteriorate due to several factors including physical and chemical effects.
One of the major causes of concrete structures deterioration today is corrosion of reinforced
steel. Occurrence of corrosion results to structural weakening, which is caused by a decrease in
cross-section of the steel, concrete spalling, concrete cracking, surface staining, and concrete
delamination. The corrosion ends up reducing the reinforced concrete (RC) structure’s service
life. The main problem associated with the safety and structural soundness or integrity of RC
structures is the reduction of its load-bearing capacity. The main purpose of this project was to
investigate the extent of corrosion and deterioration of RC structure – a bridge girder, and to
establish potential factors that contributes to deterioration of bridges in general. This was
attained by carrying out several tests and investigations including non-destructive testing and
visual inspection of the bridge.
Corrosion of Steel Reinforcement in RC Structures
Corrosion of reinforcement has a significant effect on the durability of RC structures hence it is
worth being investigated. The damage of concrete caused by corrosion is among the leading
causes of decreased durability of RC structures. A study conducted by researchers worldwide
found that steel reinforcement rusting causes more than four-hundredth of structural failures
across the world.
Effect of Reinforcement Corrosion on Structural Soundness
There are two major damaging effects associated with corrosion of steel reinforcement of
concrete structures. These are:

Corrosion and Deterioration of RC structure 3
i) Corrosion produces rust that has volume equivalent to two to four times more than the
volume of steel. The increase in volume results to a corresponding increase in
concrete’s tensile stresses that causes cracking and spalling of concrete cover. The
loss of concrete cover reduces the load bearing capacity of the RC structure and also
further exposes the steel reinforcement to the harsh environmental agents.
ii) Corrosion causes reduction of steel’s cross sectional area. This reduction in cross
sectional area of steel makes the RC structure unable to support its design loads.
Therefore corrosion of steel reinforcement does not only affect the external appearance of RC
structures but also significantly affects their structural integrity, performance, functionality and
safety.
Corrosion Mechanism of Steel Reinforcement in Concrete
Corrosion Cell
Corrosion of steel reinforced in concrete is linked to electrochemical process. The process by
which the steel corrodes is apparently similar to the process that occurs in a flash battery. In this
scenario, the chemically corroded steel surface acts as a mixed conductor comprising of cathodes
and anodes that are electrically connected via the steel itself, resulting to various reactions. The
water pores that are present in concrete acts as aqueous medium. Al these create a corrosion cell
within the reinforcement, as shown in Figure 1 below
i) Corrosion produces rust that has volume equivalent to two to four times more than the
volume of steel. The increase in volume results to a corresponding increase in
concrete’s tensile stresses that causes cracking and spalling of concrete cover. The
loss of concrete cover reduces the load bearing capacity of the RC structure and also
further exposes the steel reinforcement to the harsh environmental agents.
ii) Corrosion causes reduction of steel’s cross sectional area. This reduction in cross
sectional area of steel makes the RC structure unable to support its design loads.
Therefore corrosion of steel reinforcement does not only affect the external appearance of RC
structures but also significantly affects their structural integrity, performance, functionality and
safety.
Corrosion Mechanism of Steel Reinforcement in Concrete
Corrosion Cell
Corrosion of steel reinforced in concrete is linked to electrochemical process. The process by
which the steel corrodes is apparently similar to the process that occurs in a flash battery. In this
scenario, the chemically corroded steel surface acts as a mixed conductor comprising of cathodes
and anodes that are electrically connected via the steel itself, resulting to various reactions. The
water pores that are present in concrete acts as aqueous medium. Al these create a corrosion cell
within the reinforcement, as shown in Figure 1 below
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Corrosion and Deterioration of RC structure 4
Figure 1: Corrosion cell of steel reinforcement
Thermodynamics of Corrosion
Corrosion is understood to be an electrochemical process which takes place in the presence of
oxygen and water. Equation 1 and 2 below describes the key redox reactions of corrosion.
Equation 1 represents iron’s anodic oxidation whereas equation 2 represents oxygen’s cathodic
reduction. Equation 3 represents the general equation of corrosion, where Fe(OH)2 is just one of
the possible products that are produced during corrosion. The specific products produced depend
on the conditions present including oxygen, moisture, temperature and pH, among others.
Fe → Fe2+ + 2e- ………………………………………………………….. (1)
H2O + ½O2 + 2e- → 2OH ……………………………………………. (2)
Fe + H2O + ½O2 → Fe(OH)2 ………………………………………… (3)
The state of steel fixed in untainted concrete is typically passive as a result of the pore solution’s
high alkalinity. This leads to formation of a passive film – an iron oxide layer that acts as a
protective layer. The thermodynamic fields of corrosion, passivity and immunity of iron and iron
oxide present in the solution are shown in Pourbaix chart shown in Figure 2 below. The dashed
lines in the chart shows the equilibrium potentials of iron. Hydrogen or oxygen reduction takes
place below the dashed lines.
Figure 1: Corrosion cell of steel reinforcement
Thermodynamics of Corrosion
Corrosion is understood to be an electrochemical process which takes place in the presence of
oxygen and water. Equation 1 and 2 below describes the key redox reactions of corrosion.
Equation 1 represents iron’s anodic oxidation whereas equation 2 represents oxygen’s cathodic
reduction. Equation 3 represents the general equation of corrosion, where Fe(OH)2 is just one of
the possible products that are produced during corrosion. The specific products produced depend
on the conditions present including oxygen, moisture, temperature and pH, among others.
Fe → Fe2+ + 2e- ………………………………………………………….. (1)
H2O + ½O2 + 2e- → 2OH ……………………………………………. (2)
Fe + H2O + ½O2 → Fe(OH)2 ………………………………………… (3)
The state of steel fixed in untainted concrete is typically passive as a result of the pore solution’s
high alkalinity. This leads to formation of a passive film – an iron oxide layer that acts as a
protective layer. The thermodynamic fields of corrosion, passivity and immunity of iron and iron
oxide present in the solution are shown in Pourbaix chart shown in Figure 2 below. The dashed
lines in the chart shows the equilibrium potentials of iron. Hydrogen or oxygen reduction takes
place below the dashed lines.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
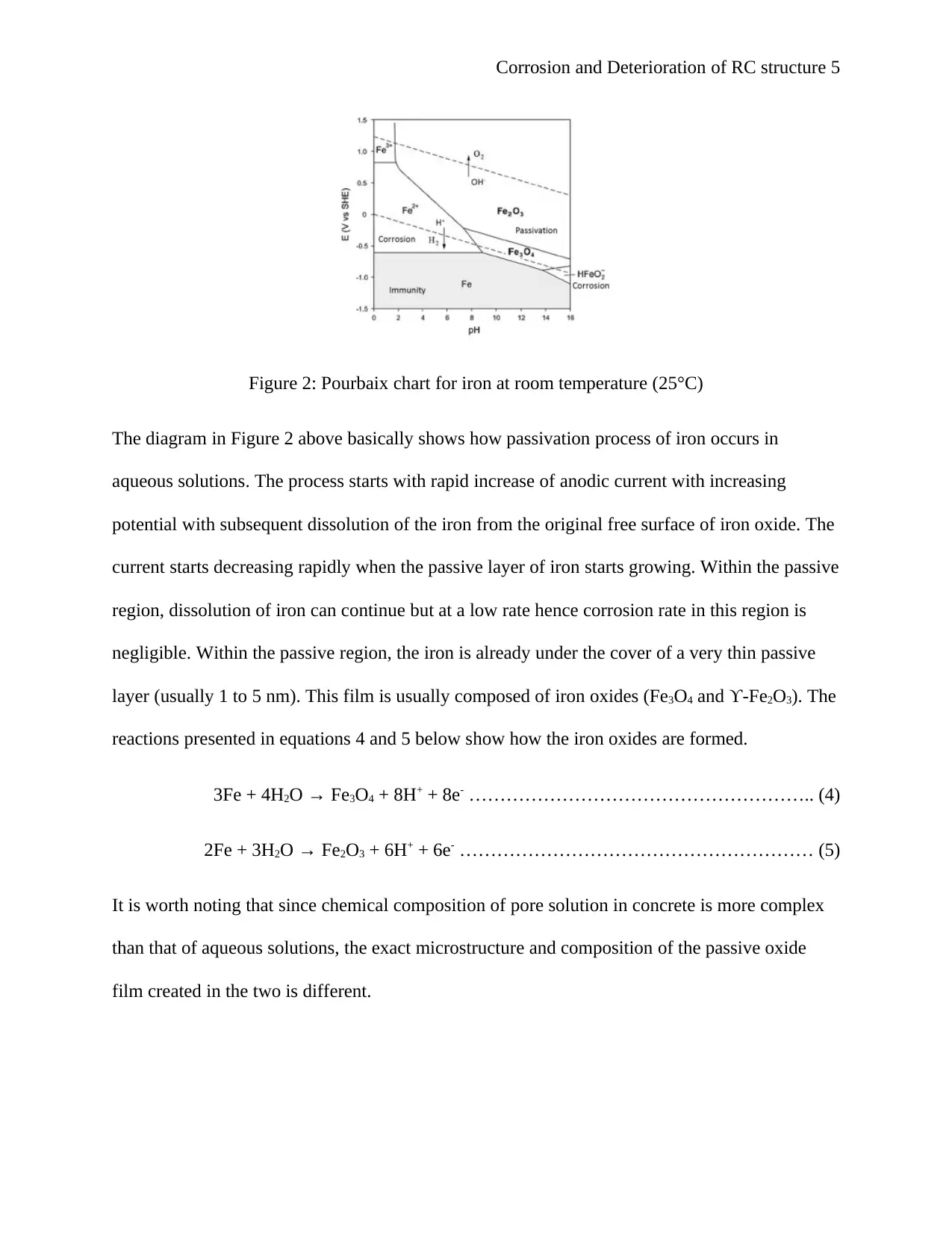
Corrosion and Deterioration of RC structure 5
Figure 2: Pourbaix chart for iron at room temperature (25°C)
The diagram in Figure 2 above basically shows how passivation process of iron occurs in
aqueous solutions. The process starts with rapid increase of anodic current with increasing
potential with subsequent dissolution of the iron from the original free surface of iron oxide. The
current starts decreasing rapidly when the passive layer of iron starts growing. Within the passive
region, dissolution of iron can continue but at a low rate hence corrosion rate in this region is
negligible. Within the passive region, the iron is already under the cover of a very thin passive
layer (usually 1 to 5 nm). This film is usually composed of iron oxides (Fe3O4 and ϒ-Fe2O3). The
reactions presented in equations 4 and 5 below show how the iron oxides are formed.
3Fe + 4H2O → Fe3O4 + 8H+ + 8e- ……………………………………………….. (4)
2Fe + 3H2O → Fe2O3 + 6H+ + 6e- ………………………………………………… (5)
It is worth noting that since chemical composition of pore solution in concrete is more complex
than that of aqueous solutions, the exact microstructure and composition of the passive oxide
film created in the two is different.
Figure 2: Pourbaix chart for iron at room temperature (25°C)
The diagram in Figure 2 above basically shows how passivation process of iron occurs in
aqueous solutions. The process starts with rapid increase of anodic current with increasing
potential with subsequent dissolution of the iron from the original free surface of iron oxide. The
current starts decreasing rapidly when the passive layer of iron starts growing. Within the passive
region, dissolution of iron can continue but at a low rate hence corrosion rate in this region is
negligible. Within the passive region, the iron is already under the cover of a very thin passive
layer (usually 1 to 5 nm). This film is usually composed of iron oxides (Fe3O4 and ϒ-Fe2O3). The
reactions presented in equations 4 and 5 below show how the iron oxides are formed.
3Fe + 4H2O → Fe3O4 + 8H+ + 8e- ……………………………………………….. (4)
2Fe + 3H2O → Fe2O3 + 6H+ + 6e- ………………………………………………… (5)
It is worth noting that since chemical composition of pore solution in concrete is more complex
than that of aqueous solutions, the exact microstructure and composition of the passive oxide
film created in the two is different.

Corrosion and Deterioration of RC structure 6
Pitting Corrosion
There are several researches that have been conducted on pitting corrosion but the manner in
which chloride ions contribute to pitting corrosion is yet to be understood fully. However
passivity breakdown is attributed to one of the following mechanisms: adsorption mechanism,
film breaking mechanism and penetration mechanism. The principle of penetration mechanism is
that the great potential difference in the passive layer causes penetration of the chloride ions
present in the electrolyte through the metal surface’s passive film. According to film breaking
mechanism, incoherence present in the passive layer allows chloride ions to reach the metal
surface directly. In adsorption mechanism, the chloride ions get adsorbed to the passive layer
resulting to progressive thinning until the end of dissolution. Details of establishing the exact
mechanism that result to breakdown of passive film is not covered in this thesis. Nevertheless,
the general process on how pitting corrosion occurs in concrete due to induced chloride ions is
represented by the schematic in Figure 3 below
Figure 3: Pitting corrosion induced by chloride
After passivity has been broken down, a pit gets created and dissolution of iron continues
(equation 1). The electrons move from the anode to cathode, where the process of oxygen
reduction occurs (equation 2). For the positive charges that are generated at the anode to be
Pitting Corrosion
There are several researches that have been conducted on pitting corrosion but the manner in
which chloride ions contribute to pitting corrosion is yet to be understood fully. However
passivity breakdown is attributed to one of the following mechanisms: adsorption mechanism,
film breaking mechanism and penetration mechanism. The principle of penetration mechanism is
that the great potential difference in the passive layer causes penetration of the chloride ions
present in the electrolyte through the metal surface’s passive film. According to film breaking
mechanism, incoherence present in the passive layer allows chloride ions to reach the metal
surface directly. In adsorption mechanism, the chloride ions get adsorbed to the passive layer
resulting to progressive thinning until the end of dissolution. Details of establishing the exact
mechanism that result to breakdown of passive film is not covered in this thesis. Nevertheless,
the general process on how pitting corrosion occurs in concrete due to induced chloride ions is
represented by the schematic in Figure 3 below
Figure 3: Pitting corrosion induced by chloride
After passivity has been broken down, a pit gets created and dissolution of iron continues
(equation 1). The electrons move from the anode to cathode, where the process of oxygen
reduction occurs (equation 2). For the positive charges that are generated at the anode to be
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Corrosion and Deterioration of RC structure 7
balanced and electro neutrality maintained, OH- and Cl- (anions) and Na+, Ca2+ and K+ (cations)
move away from and towards the cathode respectively. Within the formed pit, the dissolved iron
ions get hydrolyzed as described by equations 6, 7, 8 and 9. This hydrolysis results to
acidification.
Additionally, a porous cap comprising of passive film remnants and products of iron rust can be
formed. This significantly minimizes transport to and from the pit. When the environment is
alkaline, hydroxyl ions will move inside the pit for the formation of H+ to be balance (equations
6, 7 and 8). When migration of chloride ions creates an acid environment in the pit, hydrochloric
acid may be formed (equation 9). Considering the low pH in the pit, occurrence of cathodic
hydrogen evolution is possible. This means that the pit will act as a cathode and anode resulting
to occurrence of hydrogen embrittlement of reinforced steel.
Fe2+ + 2H2O → Fe(OH)2 + 2H+ ………………………………………………. (6)
2Fe2+ + 3H2O → Fe2O3 + 6H+ + 2e- ………………………………………… (7)
3Fe2+ + 4H2O → Fe3O4 + 8H+ + 2e- ………………………………………… (8)
3Fe2+ + 4H2O → Fe3O4 + 8H+ + 2e- ……………………………………….. (9)
A number of studies have also revealed formation of green rust (soluble iron chloride complexes)
represented as FeCl2. Green rusts are basically a group of FeCl2 compounds where Cl-, Fe2+ and
Fe3+ are present and only stable in environments that are deprived of oxygen. It is believed that
the mechanism results to production of these compounds inside the pit, which then diffuses to
oxygen and hydroxide richer regions and creation of solid rust products. This also produces
chloride ions that attacks the passive film even more. This catalytic effect also produces acid (as
shown in equation 10) that is aggressive to steel.
balanced and electro neutrality maintained, OH- and Cl- (anions) and Na+, Ca2+ and K+ (cations)
move away from and towards the cathode respectively. Within the formed pit, the dissolved iron
ions get hydrolyzed as described by equations 6, 7, 8 and 9. This hydrolysis results to
acidification.
Additionally, a porous cap comprising of passive film remnants and products of iron rust can be
formed. This significantly minimizes transport to and from the pit. When the environment is
alkaline, hydroxyl ions will move inside the pit for the formation of H+ to be balance (equations
6, 7 and 8). When migration of chloride ions creates an acid environment in the pit, hydrochloric
acid may be formed (equation 9). Considering the low pH in the pit, occurrence of cathodic
hydrogen evolution is possible. This means that the pit will act as a cathode and anode resulting
to occurrence of hydrogen embrittlement of reinforced steel.
Fe2+ + 2H2O → Fe(OH)2 + 2H+ ………………………………………………. (6)
2Fe2+ + 3H2O → Fe2O3 + 6H+ + 2e- ………………………………………… (7)
3Fe2+ + 4H2O → Fe3O4 + 8H+ + 2e- ………………………………………… (8)
3Fe2+ + 4H2O → Fe3O4 + 8H+ + 2e- ……………………………………….. (9)
A number of studies have also revealed formation of green rust (soluble iron chloride complexes)
represented as FeCl2. Green rusts are basically a group of FeCl2 compounds where Cl-, Fe2+ and
Fe3+ are present and only stable in environments that are deprived of oxygen. It is believed that
the mechanism results to production of these compounds inside the pit, which then diffuses to
oxygen and hydroxide richer regions and creation of solid rust products. This also produces
chloride ions that attacks the passive film even more. This catalytic effect also produces acid (as
shown in equation 10) that is aggressive to steel.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Corrosion and Deterioration of RC structure 8
4FeCl2 + O2 + 6H2O → 4FeOOH + 8HCl …………………………………………….. (10)
Factors Affecting Concrete Steel Corrosion
Factors that affect corrosion of steel fixed in concrete can be categorized into two main classes:
internal factors and external factors.
External factors
External factors are mainly environmental conditions and they include the following:
i) Moisture and oxygen
Corrosion is facilitated by the presence of oxygen and moisture. Presence of moisture meets the
requirement of electrolytic process of the corrosion cell, and both oxygen and moisture facilitates
formation of hydroxide (OH) that produces Fe(OH)2 – a component of rust. The presence of
oxygen facilitates cathodic reactions but corrosion cannot progress in the absence of adequate
oxygen because of cathodic polarization.
ii) Temperature and relative humidity
Carbonation of concrete is significantly affected by relative humidly. An increase in relative
humidity results to a decrease in concrete carbonation when the relative humidity in within the
range of 50-100%. A study revealed that when relative humidity is within 30-50%, a decrease in
relative humidity does not result to a corresponding decrease in concrete carbonation especially
when CO2 concentration is normal even when the steel is exposed for a longer period of time. On
the other hand, an increase in temperature can result to two effects: first is an increase in the rates
of electrode reaction, and second is a decrease in solubility of oxygen, which reduces the rate of
corrosion. If there is a conducive environment for the occurrence of corrosion, high humidity and
high temperature results to an increase in the corrosion rate.
4FeCl2 + O2 + 6H2O → 4FeOOH + 8HCl …………………………………………….. (10)
Factors Affecting Concrete Steel Corrosion
Factors that affect corrosion of steel fixed in concrete can be categorized into two main classes:
internal factors and external factors.
External factors
External factors are mainly environmental conditions and they include the following:
i) Moisture and oxygen
Corrosion is facilitated by the presence of oxygen and moisture. Presence of moisture meets the
requirement of electrolytic process of the corrosion cell, and both oxygen and moisture facilitates
formation of hydroxide (OH) that produces Fe(OH)2 – a component of rust. The presence of
oxygen facilitates cathodic reactions but corrosion cannot progress in the absence of adequate
oxygen because of cathodic polarization.
ii) Temperature and relative humidity
Carbonation of concrete is significantly affected by relative humidly. An increase in relative
humidity results to a decrease in concrete carbonation when the relative humidity in within the
range of 50-100%. A study revealed that when relative humidity is within 30-50%, a decrease in
relative humidity does not result to a corresponding decrease in concrete carbonation especially
when CO2 concentration is normal even when the steel is exposed for a longer period of time. On
the other hand, an increase in temperature can result to two effects: first is an increase in the rates
of electrode reaction, and second is a decrease in solubility of oxygen, which reduces the rate of
corrosion. If there is a conducive environment for the occurrence of corrosion, high humidity and
high temperature results to an increase in the corrosion rate.

Corrosion and Deterioration of RC structure 9
iii) Carbonation and acidic gaseous pollutants
Carbonation and acidic gases like NO2 and SO2 affects corrosion because of the ability to
decrease concrete’s pH. When the pH level reduces to a certain range, reinforcement corrosion
may start, in addition to loss of concrete passivity and catastrophic corrosion of the
reinforcement, as shown in Table 1 below.
Table 1: Reinforcement corrosion state at different pH levels
iv) Aggressive anions
The main aggressive anions are chloride ions that can reach the reinforcement level from the
external environment or via the ingredients of concrete. There are three forms in which chloride
can be present in concrete: acid soluble chloride, bound chloride and water-soluble or free
chloride. The corrosion process is only influenced by the free chloride ions. Generally, an
increase in chloride content results to a decrease in resistivity and an increase in the rate of
corrosion. Nevertheless, a change in pH is deemed to have an insignificant effect on the
corrosion rate because of a change in the concrete’s chloride content. Table 2 below shows the
risk of reinforcement in association with chloride content levels in carbonated and uncarbonated
concrete.
Table 2: Risk of corrosion in concrete with chloride
iii) Carbonation and acidic gaseous pollutants
Carbonation and acidic gases like NO2 and SO2 affects corrosion because of the ability to
decrease concrete’s pH. When the pH level reduces to a certain range, reinforcement corrosion
may start, in addition to loss of concrete passivity and catastrophic corrosion of the
reinforcement, as shown in Table 1 below.
Table 1: Reinforcement corrosion state at different pH levels
iv) Aggressive anions
The main aggressive anions are chloride ions that can reach the reinforcement level from the
external environment or via the ingredients of concrete. There are three forms in which chloride
can be present in concrete: acid soluble chloride, bound chloride and water-soluble or free
chloride. The corrosion process is only influenced by the free chloride ions. Generally, an
increase in chloride content results to a decrease in resistivity and an increase in the rate of
corrosion. Nevertheless, a change in pH is deemed to have an insignificant effect on the
corrosion rate because of a change in the concrete’s chloride content. Table 2 below shows the
risk of reinforcement in association with chloride content levels in carbonated and uncarbonated
concrete.
Table 2: Risk of corrosion in concrete with chloride
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Corrosion and Deterioration of RC structure 10
Several countries have also come up with codes recommending limits of total chloride ion
content that should be contained in concrete, as shown in Table 3 below.
Table 3: Recommended concrete chloride content limits
v) Bacteria
There are three different ways in which bacteria action is effective in facilitating corrosion.
These are:
a) Bacteria reduces the amount of concrete cover through fragmentation of cementitious
materials.
b) In oxygen deprived environment, anaerobic bacteria generates iron sulfides that
facilitates corrosion reaction.
Several countries have also come up with codes recommending limits of total chloride ion
content that should be contained in concrete, as shown in Table 3 below.
Table 3: Recommended concrete chloride content limits
v) Bacteria
There are three different ways in which bacteria action is effective in facilitating corrosion.
These are:
a) Bacteria reduces the amount of concrete cover through fragmentation of cementitious
materials.
b) In oxygen deprived environment, anaerobic bacteria generates iron sulfides that
facilitates corrosion reaction.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Corrosion and Deterioration of RC structure 11
c) Aerobic bacteria also facilitate creation of differential aeration cells than may lead to
corrosion.
Internal factors
The internal factors mainly comprise of factors associated with the quality of steel and concrete.
These factors include the following:
i) Cement composition
There are two main ways in which the cement present in concrete protects the reinforcing steel
from corrosion. First is by sustaining a high pH ranging between 12.5 and 13. This is due to the
presence of alkaline materials such as Ca(OH)2 that are produced when cement get hydrated.
Second is by binding a substantial quantity of total chlorides due to chemical reaction between
C4AF and C3A content present in cement inside the concrete. As a result, an increase in the
content of C3A in the cement causes the value of chloride to move to the higher side. Blended
cement, like micro-silica, which has high content of C3A, significantly increases resistance to
chloride corrosion and sulfate attack on reinforcement.
ii) Aggregate impurities
When the aggregates used contain chloride salts, serious corrosion problems arise. This is very
common in areas close to the sea and those sites whose groundwater has high chloride ions
concentration.
iii) Mixing and curing water impurities
When water used for mixing or curing concrete is contaminated with high chloride content or
significantly acidified, serious corrosion problems arise.
c) Aerobic bacteria also facilitate creation of differential aeration cells than may lead to
corrosion.
Internal factors
The internal factors mainly comprise of factors associated with the quality of steel and concrete.
These factors include the following:
i) Cement composition
There are two main ways in which the cement present in concrete protects the reinforcing steel
from corrosion. First is by sustaining a high pH ranging between 12.5 and 13. This is due to the
presence of alkaline materials such as Ca(OH)2 that are produced when cement get hydrated.
Second is by binding a substantial quantity of total chlorides due to chemical reaction between
C4AF and C3A content present in cement inside the concrete. As a result, an increase in the
content of C3A in the cement causes the value of chloride to move to the higher side. Blended
cement, like micro-silica, which has high content of C3A, significantly increases resistance to
chloride corrosion and sulfate attack on reinforcement.
ii) Aggregate impurities
When the aggregates used contain chloride salts, serious corrosion problems arise. This is very
common in areas close to the sea and those sites whose groundwater has high chloride ions
concentration.
iii) Mixing and curing water impurities
When water used for mixing or curing concrete is contaminated with high chloride content or
significantly acidified, serious corrosion problems arise.

Corrosion and Deterioration of RC structure 12
iv) Admixtures
Calcium chloride is a very common admixture that is usually added to concrete to speed up
hydration of cement. However, this chloride content is detrimental to RC structures because it
facilitates corrosion of steel reinforcement.
v) Water-cement (w/c) ratio
W/c ratio principals affects the strength, workability, impermeability and durability of concrete.
This parameter does not directly affect corrosion rate in reinforcement. However, when a RC
structure comes in contact with an aggressive solution, the concrete’s permeability, which is
affected by the w/c ratio, affects the rebar’s corrosion. The w/c ratio is directly proportional to
the penetration depth of a certain value of chloride threshold. It has been found that carbonation
depth increases linearly with the increase in w/c ratio. Also, it has been found that an increase in
the w/c ratio results to a corresponding increase in the coefficient of oxygen diffusion, and vice
versa.
vi) Cement content
Concrete’s cement content affects various parameters including the strength and durability of
concrete. When the amount of cement used is inadequate, surface defects such as honeycombs
get formed in the concrete. These defects allow corrosion causing agents like O2, CO2, H2O and
Cl- to penetrate and diffuse into the concrete. These agents facilitate creation of differential cells
that initiate corrosion of reinforcement. Additionally, concrete that has low cement content lacks
plastic consistency hence it is not able to create a uniform passive film to cover and protect the
rebars, which creates loopholes for corrosion.
vii) Concrete cover
iv) Admixtures
Calcium chloride is a very common admixture that is usually added to concrete to speed up
hydration of cement. However, this chloride content is detrimental to RC structures because it
facilitates corrosion of steel reinforcement.
v) Water-cement (w/c) ratio
W/c ratio principals affects the strength, workability, impermeability and durability of concrete.
This parameter does not directly affect corrosion rate in reinforcement. However, when a RC
structure comes in contact with an aggressive solution, the concrete’s permeability, which is
affected by the w/c ratio, affects the rebar’s corrosion. The w/c ratio is directly proportional to
the penetration depth of a certain value of chloride threshold. It has been found that carbonation
depth increases linearly with the increase in w/c ratio. Also, it has been found that an increase in
the w/c ratio results to a corresponding increase in the coefficient of oxygen diffusion, and vice
versa.
vi) Cement content
Concrete’s cement content affects various parameters including the strength and durability of
concrete. When the amount of cement used is inadequate, surface defects such as honeycombs
get formed in the concrete. These defects allow corrosion causing agents like O2, CO2, H2O and
Cl- to penetrate and diffuse into the concrete. These agents facilitate creation of differential cells
that initiate corrosion of reinforcement. Additionally, concrete that has low cement content lacks
plastic consistency hence it is not able to create a uniform passive film to cover and protect the
rebars, which creates loopholes for corrosion.
vii) Concrete cover
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 29
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




