Report on Factors Affecting Reaction Rate: Kinetics of MgCO3 & HNO3
VerifiedAdded on 2022/08/21
|11
|1747
|35
Report
AI Summary
This report investigates the factors influencing the reaction rate between magnesium carbonate and dilute nitric acid, focusing on temperature, concentration, surface area, and the presence of a catalyst. Experimental results demonstrate that increasing temperature and reactant concentration accelerates the reaction rate, as explained by collision theory and Maxwell-Boltzmann distributions. A larger surface area, achieved by using powdered magnesium carbonate, also enhances the reaction rate due to increased collision frequency. The inclusion of a catalyst lowers the activation energy, further speeding up the reaction. The report concludes that these factors directly impact the rate of chemical reactions, providing a comprehensive understanding of reaction kinetics. Desklib provides a wide range of solved assignments and past papers for students.

Reaction kinetics 1
FACTORS AFFECTING REACTION
by (Name)
Course
Instructor
University
City
Date
FACTORS AFFECTING REACTION
by (Name)
Course
Instructor
University
City
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Reaction kinetics 2
ABSTRACT
Chemical kinetics, which is also called reaction kinetics is the study of chemical processes.
These are not limited to the investigation of how various experimental situations can affect the
speed of a chemical reaction and it products. Chemical reactions differ greatly in quickness at
which they take place. Some are instantaneous while others take ages to come to attain
equilibrium position. The experiment is conducted to confirm the factors affecting reaction rate.
ABSTRACT
Chemical kinetics, which is also called reaction kinetics is the study of chemical processes.
These are not limited to the investigation of how various experimental situations can affect the
speed of a chemical reaction and it products. Chemical reactions differ greatly in quickness at
which they take place. Some are instantaneous while others take ages to come to attain
equilibrium position. The experiment is conducted to confirm the factors affecting reaction rate.
Reaction kinetics 3
FACTORS AFFECTING REACTION RATE
Introduction
Rate of a reaction is a measure of how fast or slow a reaction occurs. To be able to
determine how a reaction progresses, time taken for reaction reactants to be completely
consumed over a duration of time.
Reaction rate= quantity of substance change / time for change to occur
Hence, reaction rate is a rate of change of an amount or concentration of a particular reactant per
unit time. A number of aspects which affect reaction rate; reacting species concentration ,
temperature, reactants surface area and the catalyst.
EXPERIMENT TO INVESTIGATE FACTORS AFFECTING REACTION OF MAGNESIUM
CARBONATE AND DILUTE NITRIC ACID.
Results and discussion
Results
Reactants
With a catalyst
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 40 30
Lumps of metal carbonate, 2 moldm-3 acid 80 60
Powdered metal carbonate, 1 moldm-3 acid 60 50
Lumps of metal carbonate, 1 moldm-3 acid 120 100
Without a catalyst
FACTORS AFFECTING REACTION RATE
Introduction
Rate of a reaction is a measure of how fast or slow a reaction occurs. To be able to
determine how a reaction progresses, time taken for reaction reactants to be completely
consumed over a duration of time.
Reaction rate= quantity of substance change / time for change to occur
Hence, reaction rate is a rate of change of an amount or concentration of a particular reactant per
unit time. A number of aspects which affect reaction rate; reacting species concentration ,
temperature, reactants surface area and the catalyst.
EXPERIMENT TO INVESTIGATE FACTORS AFFECTING REACTION OF MAGNESIUM
CARBONATE AND DILUTE NITRIC ACID.
Results and discussion
Results
Reactants
With a catalyst
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 40 30
Lumps of metal carbonate, 2 moldm-3 acid 80 60
Powdered metal carbonate, 1 moldm-3 acid 60 50
Lumps of metal carbonate, 1 moldm-3 acid 120 100
Without a catalyst
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Reaction kinetics 4
Reactants
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 80 60
Lumps of metal carbonate, 2 moldm-3 acid 160 120
Powdered metal carbonate, 1 moldm-3 acid 120 100
Lumps of metal carbonate, 1 moldm-3 acid 240 200
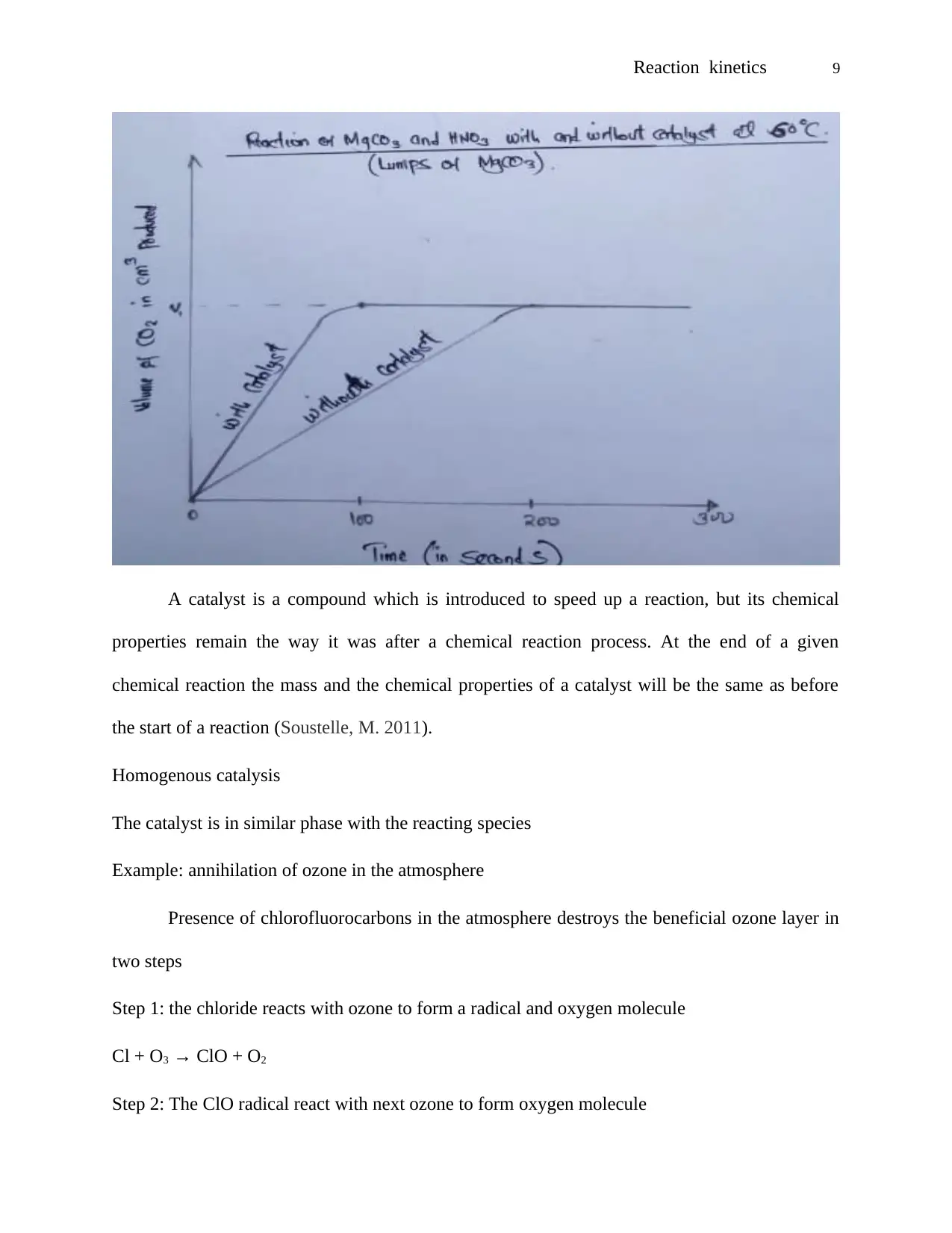
Discussions
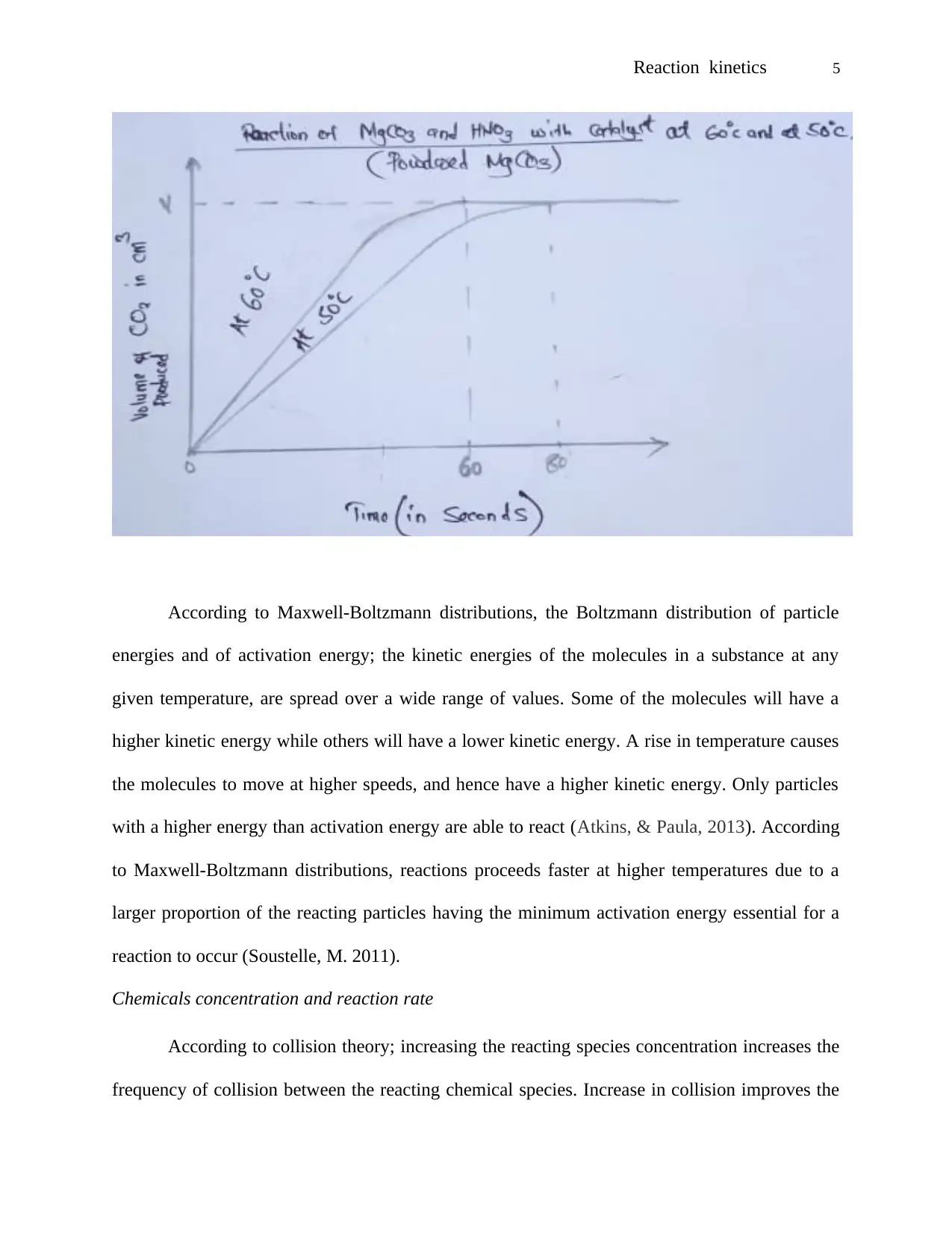
Influence of temperature on reaction rate
When equal powdered magnesium carbonate was introduced in heated nitric acid with a
temperature of 60 °C, it took relatively shorter time for carbon IV oxide gas to be collected than
when it was introduced in heated nitric acid with a temperature of 50 °C.
Illustration of a chemical reaction
MgCO3 (s) + HNO3 (aq.) → Mg(NO3)2 (aq.) + H2O (l) + CO2 (aq.)
According to collision theory, temperature rise escalates the kinetic energy of the reacting
species (MgCO3 (s) and HNO3 (aq.)) which in turn increases the incidence of collision of the
reacting species. The escalation in kinetic energy of the colliding particles provides the needed
activation energy necessary for the reaction to occur. A rise in temperature therefore escalates
the rate of reaction, making it to proceed faster (Laidler, J. 2016).
Figure 1: showing the influence of temperature on reaction rate
Reactants
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 80 60
Lumps of metal carbonate, 2 moldm-3 acid 160 120
Powdered metal carbonate, 1 moldm-3 acid 120 100
Lumps of metal carbonate, 1 moldm-3 acid 240 200
Discussions
Influence of temperature on reaction rate
When equal powdered magnesium carbonate was introduced in heated nitric acid with a
temperature of 60 °C, it took relatively shorter time for carbon IV oxide gas to be collected than
when it was introduced in heated nitric acid with a temperature of 50 °C.
Illustration of a chemical reaction
MgCO3 (s) + HNO3 (aq.) → Mg(NO3)2 (aq.) + H2O (l) + CO2 (aq.)
According to collision theory, temperature rise escalates the kinetic energy of the reacting
species (MgCO3 (s) and HNO3 (aq.)) which in turn increases the incidence of collision of the
reacting species. The escalation in kinetic energy of the colliding particles provides the needed
activation energy necessary for the reaction to occur. A rise in temperature therefore escalates
the rate of reaction, making it to proceed faster (Laidler, J. 2016).
Figure 1: showing the influence of temperature on reaction rate
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Reaction kinetics 5
According to Maxwell-Boltzmann distributions, the Boltzmann distribution of particle
energies and of activation energy; the kinetic energies of the molecules in a substance at any
given temperature, are spread over a wide range of values. Some of the molecules will have a
higher kinetic energy while others will have a lower kinetic energy. A rise in temperature causes
the molecules to move at higher speeds, and hence have a higher kinetic energy. Only particles
with a higher energy than activation energy are able to react (Atkins, & Paula, 2013). According
to Maxwell-Boltzmann distributions, reactions proceeds faster at higher temperatures due to a
larger proportion of the reacting particles having the minimum activation energy essential for a
reaction to occur (Soustelle, M. 2011).
Chemicals concentration and reaction rate
According to collision theory; increasing the reacting species concentration increases the
frequency of collision between the reacting chemical species. Increase in collision improves the
According to Maxwell-Boltzmann distributions, the Boltzmann distribution of particle
energies and of activation energy; the kinetic energies of the molecules in a substance at any
given temperature, are spread over a wide range of values. Some of the molecules will have a
higher kinetic energy while others will have a lower kinetic energy. A rise in temperature causes
the molecules to move at higher speeds, and hence have a higher kinetic energy. Only particles
with a higher energy than activation energy are able to react (Atkins, & Paula, 2013). According
to Maxwell-Boltzmann distributions, reactions proceeds faster at higher temperatures due to a
larger proportion of the reacting particles having the minimum activation energy essential for a
reaction to occur (Soustelle, M. 2011).
Chemicals concentration and reaction rate
According to collision theory; increasing the reacting species concentration increases the
frequency of collision between the reacting chemical species. Increase in collision improves the
Reaction kinetics 6
possibility of successful collision, which may result in a chemical reaction. Increasing the
chemical concentration therefore increases the rate of a chemical reaction (Brown, et al. 2015).
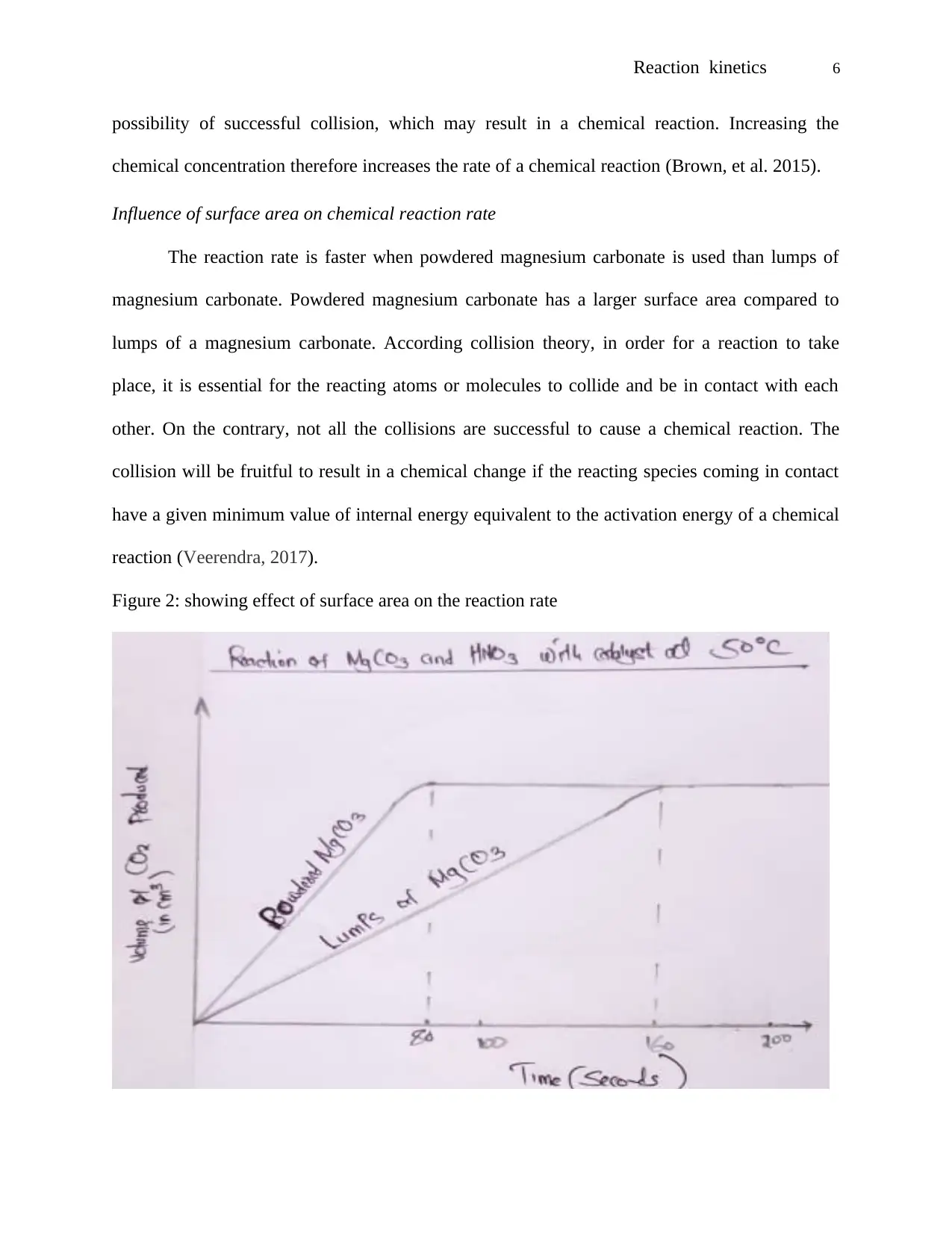
Influence of surface area on chemical reaction rate
The reaction rate is faster when powdered magnesium carbonate is used than lumps of
magnesium carbonate. Powdered magnesium carbonate has a larger surface area compared to
lumps of a magnesium carbonate. According collision theory, in order for a reaction to take
place, it is essential for the reacting atoms or molecules to collide and be in contact with each
other. On the contrary, not all the collisions are successful to cause a chemical reaction. The
collision will be fruitful to result in a chemical change if the reacting species coming in contact
have a given minimum value of internal energy equivalent to the activation energy of a chemical
reaction (Veerendra, 2017).
Figure 2: showing effect of surface area on the reaction rate
possibility of successful collision, which may result in a chemical reaction. Increasing the
chemical concentration therefore increases the rate of a chemical reaction (Brown, et al. 2015).
Influence of surface area on chemical reaction rate
The reaction rate is faster when powdered magnesium carbonate is used than lumps of
magnesium carbonate. Powdered magnesium carbonate has a larger surface area compared to
lumps of a magnesium carbonate. According collision theory, in order for a reaction to take
place, it is essential for the reacting atoms or molecules to collide and be in contact with each
other. On the contrary, not all the collisions are successful to cause a chemical reaction. The
collision will be fruitful to result in a chemical change if the reacting species coming in contact
have a given minimum value of internal energy equivalent to the activation energy of a chemical
reaction (Veerendra, 2017).
Figure 2: showing effect of surface area on the reaction rate
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Reaction kinetics 7
Moreover, the colliding chemical particles must be aligned in a way which favour the
required reorganization of atoms and electrons. Therefore, as per the collision theory, the rate at
which the chemical reaction happens is equivalent to the frequency of fruitful collision
(Veerendra, 2017).
In a chemical reaction the reaction happens at the periphery of the reacting species. The
lump of metal carbonate has most of its particles locked up inside, hence, most of its particles are
not able to come in contact with nitric acid. Unlike a powdered metal carbonate that has a larger
surface area because it has small particles. The large surface area increases the chances of the
fruitful collisions between the reacting particles which in turn leads to an increase in the reaction
rate (Ašperger, S. 2013.). Therefore, the experiment confirms and shows that reaction rate rise
with surface area increase.
Usage of a catalyst in a chemical reaction
The colliding chemical particles need a minimum energy for a reaction to proceed. This
minimum energy necessary for reaction to proceed is called activation energy. The collisions
only results to a chemical reaction if the particles attain activation energy (Muller, P. 2016). Only
colliding chemical species with activation energy will react when they collide, those which have
a lower energy will rebound after collision. If there are very limited particles with adequate
energy at a given time, then a chemical reaction will be slower (Gold, V. 2016.).
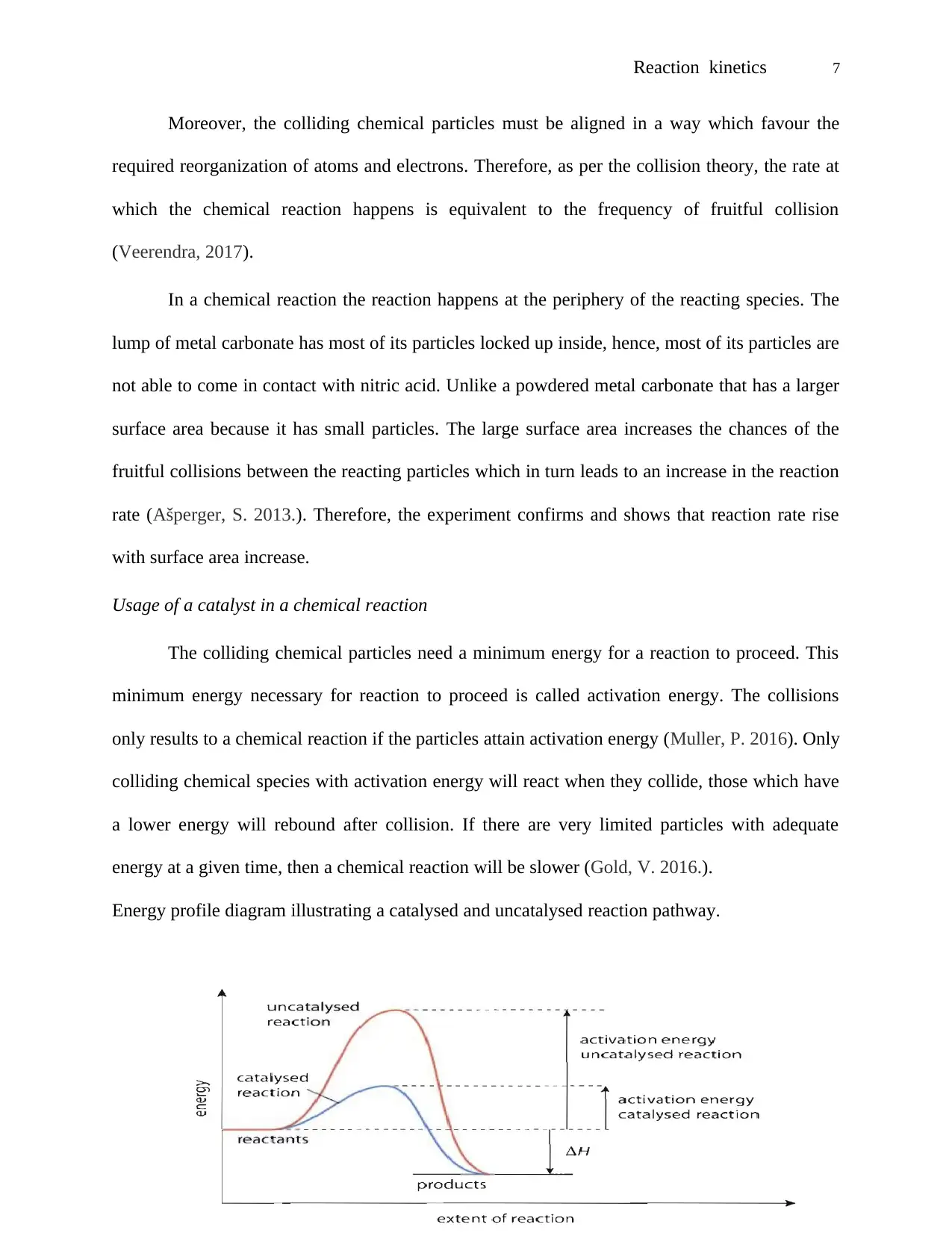
Energy profile diagram illustrating a catalysed and uncatalysed reaction pathway.
Moreover, the colliding chemical particles must be aligned in a way which favour the
required reorganization of atoms and electrons. Therefore, as per the collision theory, the rate at
which the chemical reaction happens is equivalent to the frequency of fruitful collision
(Veerendra, 2017).
In a chemical reaction the reaction happens at the periphery of the reacting species. The
lump of metal carbonate has most of its particles locked up inside, hence, most of its particles are
not able to come in contact with nitric acid. Unlike a powdered metal carbonate that has a larger
surface area because it has small particles. The large surface area increases the chances of the
fruitful collisions between the reacting particles which in turn leads to an increase in the reaction
rate (Ašperger, S. 2013.). Therefore, the experiment confirms and shows that reaction rate rise
with surface area increase.
Usage of a catalyst in a chemical reaction
The colliding chemical particles need a minimum energy for a reaction to proceed. This
minimum energy necessary for reaction to proceed is called activation energy. The collisions
only results to a chemical reaction if the particles attain activation energy (Muller, P. 2016). Only
colliding chemical species with activation energy will react when they collide, those which have
a lower energy will rebound after collision. If there are very limited particles with adequate
energy at a given time, then a chemical reaction will be slower (Gold, V. 2016.).
Energy profile diagram illustrating a catalysed and uncatalysed reaction pathway.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Reaction kinetics 8
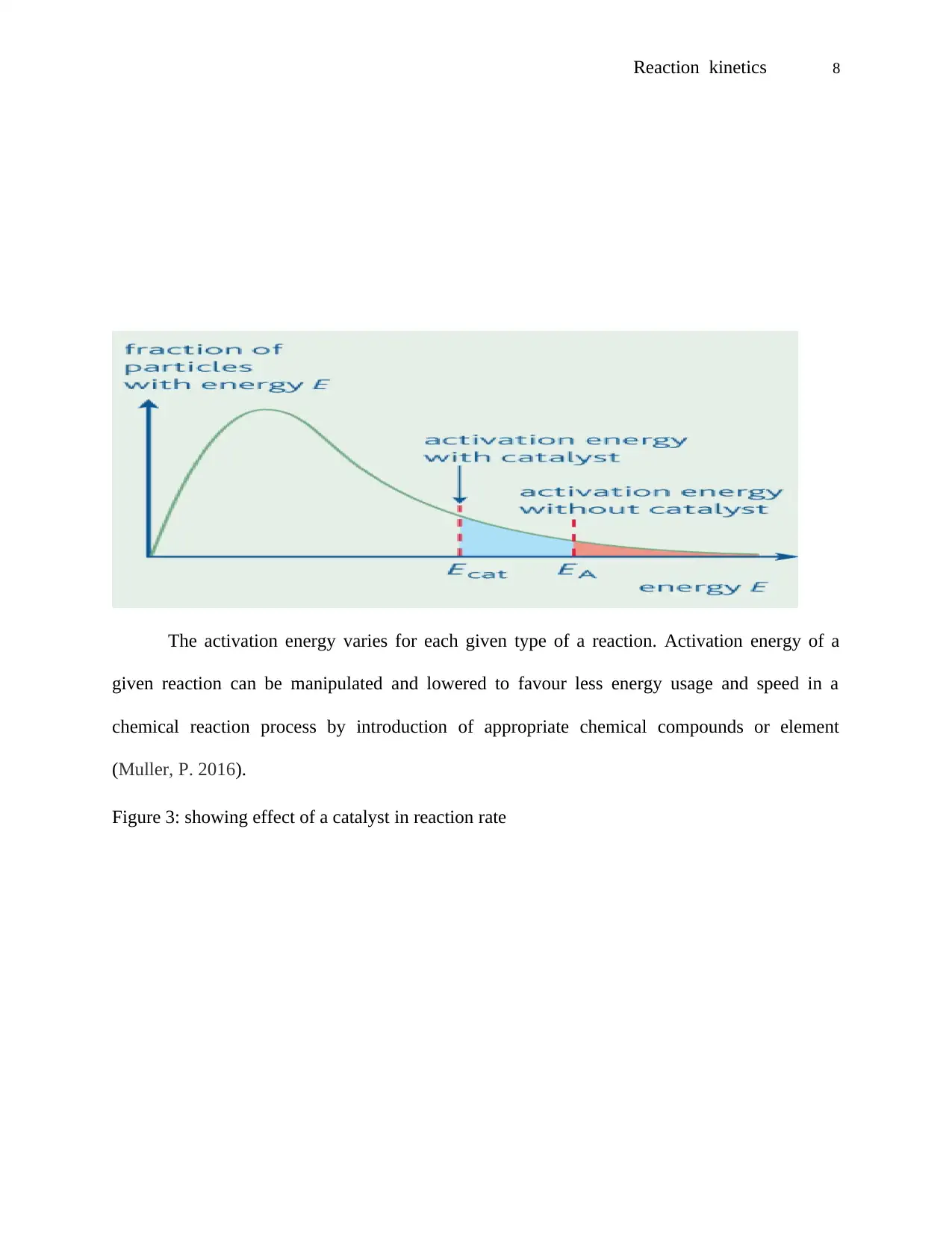
The activation energy varies for each given type of a reaction. Activation energy of a
given reaction can be manipulated and lowered to favour less energy usage and speed in a
chemical reaction process by introduction of appropriate chemical compounds or element
(Muller, P. 2016).
Figure 3: showing effect of a catalyst in reaction rate
The activation energy varies for each given type of a reaction. Activation energy of a
given reaction can be manipulated and lowered to favour less energy usage and speed in a
chemical reaction process by introduction of appropriate chemical compounds or element
(Muller, P. 2016).
Figure 3: showing effect of a catalyst in reaction rate
Reaction kinetics 9
A catalyst is a compound which is introduced to speed up a reaction, but its chemical
properties remain the way it was after a chemical reaction process. At the end of a given
chemical reaction the mass and the chemical properties of a catalyst will be the same as before
the start of a reaction (Soustelle, M. 2011).
Homogenous catalysis
The catalyst is in similar phase with the reacting species
Example: annihilation of ozone in the atmosphere
Presence of chlorofluorocarbons in the atmosphere destroys the beneficial ozone layer in
two steps
Step 1: the chloride reacts with ozone to form a radical and oxygen molecule
Cl + O3 → ClO + O2
Step 2: The ClO radical react with next ozone to form oxygen molecule
A catalyst is a compound which is introduced to speed up a reaction, but its chemical
properties remain the way it was after a chemical reaction process. At the end of a given
chemical reaction the mass and the chemical properties of a catalyst will be the same as before
the start of a reaction (Soustelle, M. 2011).
Homogenous catalysis
The catalyst is in similar phase with the reacting species
Example: annihilation of ozone in the atmosphere
Presence of chlorofluorocarbons in the atmosphere destroys the beneficial ozone layer in
two steps
Step 1: the chloride reacts with ozone to form a radical and oxygen molecule
Cl + O3 → ClO + O2
Step 2: The ClO radical react with next ozone to form oxygen molecule
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Reaction kinetics 10
ClO + O3 → Cl. + 2O2
Heterogeneous catalysis
The catalyst is in dissimilar phase with the reacting species.
Example: Hydrogenation of carbo-carbon chain double bond.
A reaction of ethane gas and hydrogen gas on the surface of nickel metal which act as a
catalyst. Both the two gases are adsorbed on the surface of the metal. The bonds between the
hydrogen molecules are broken down on the nickel surface, and the individual atoms establish
new bond with the carbon atoms. The products are then released from the surface of the nickel.
(Wright, R. 2014).
Conclusion
It is evident that there is a direct relationship between factors affecting the rate of a chemical
reaction. The surface area of the reacting particles, reactants concentration and temperature
increment results in a positive increment on the rate of reaction, and vice versa is also true. The
use of a catalyst has a positive increase too, but not in a quantifiable manner.
ClO + O3 → Cl. + 2O2
Heterogeneous catalysis
The catalyst is in dissimilar phase with the reacting species.
Example: Hydrogenation of carbo-carbon chain double bond.
A reaction of ethane gas and hydrogen gas on the surface of nickel metal which act as a
catalyst. Both the two gases are adsorbed on the surface of the metal. The bonds between the
hydrogen molecules are broken down on the nickel surface, and the individual atoms establish
new bond with the carbon atoms. The products are then released from the surface of the nickel.
(Wright, R. 2014).
Conclusion
It is evident that there is a direct relationship between factors affecting the rate of a chemical
reaction. The surface area of the reacting particles, reactants concentration and temperature
increment results in a positive increment on the rate of reaction, and vice versa is also true. The
use of a catalyst has a positive increase too, but not in a quantifiable manner.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Reaction kinetics 11
References
Atkins, P.W. & Paula, J.D., 2013. Elements of physical chemistry, Oxford: Oxford University
Press
Ašperger, S., 2013. Chemical Kinetics and Reaction Mechanisms. Chemical Kinetics and
Inorganic Reaction Mechanisms, pp.3–103.
Brown, T.L. et al., 2015. Chemistry: the central science, Boston: Pearson.
Gold, V., 2016. Energy of Activation, E a (Arrhenius Energy of Activation; Activation
Energy). IUPAC Standards Online.
Laidler, K.J., 2016. Collision Theory. IUPAC Standards Online.
Muller, P., 2016. Energy of Activation (Arrhenius Energy of Activation; Activation
Energy). IUPAC Standards Online.
Anon, Reaction Kinetics - University of Oxford. Available at:
http://vallance.chem.ox.ac.uk/pdfs/KineticsLectureNotes.pdf [Accessed January 25, 2020].
Soustelle, M., 2011. An introduction to chemical kinetics, London: ISTE.
Anon, Study.com. Available at: https://study.com/academy/lesson/the-boltzmann-distribution-
temperature-and-kinetic-energy-of-gases.html [Accessed January 25, 2020].
Veerendra, 2017. How does the surface area affect the rate of reaction? A Plus Topper. Available
at: https://www.aplustopper.com/surface-area-affect-rate-reaction/ [Accessed January 25, 2020].
Wright, M.R., 2014. Reaction kinetics, Cambridge: Royal Society of Chemistry.
References
Atkins, P.W. & Paula, J.D., 2013. Elements of physical chemistry, Oxford: Oxford University
Press
Ašperger, S., 2013. Chemical Kinetics and Reaction Mechanisms. Chemical Kinetics and
Inorganic Reaction Mechanisms, pp.3–103.
Brown, T.L. et al., 2015. Chemistry: the central science, Boston: Pearson.
Gold, V., 2016. Energy of Activation, E a (Arrhenius Energy of Activation; Activation
Energy). IUPAC Standards Online.
Laidler, K.J., 2016. Collision Theory. IUPAC Standards Online.
Muller, P., 2016. Energy of Activation (Arrhenius Energy of Activation; Activation
Energy). IUPAC Standards Online.
Anon, Reaction Kinetics - University of Oxford. Available at:
http://vallance.chem.ox.ac.uk/pdfs/KineticsLectureNotes.pdf [Accessed January 25, 2020].
Soustelle, M., 2011. An introduction to chemical kinetics, London: ISTE.
Anon, Study.com. Available at: https://study.com/academy/lesson/the-boltzmann-distribution-
temperature-and-kinetic-energy-of-gases.html [Accessed January 25, 2020].
Veerendra, 2017. How does the surface area affect the rate of reaction? A Plus Topper. Available
at: https://www.aplustopper.com/surface-area-affect-rate-reaction/ [Accessed January 25, 2020].
Wright, M.R., 2014. Reaction kinetics, Cambridge: Royal Society of Chemistry.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
