Reaction Rates and Equilibrium: A Comprehensive Chemistry Homework
VerifiedAdded on 2023/05/28
|5
|777
|381
Homework Assignment
AI Summary
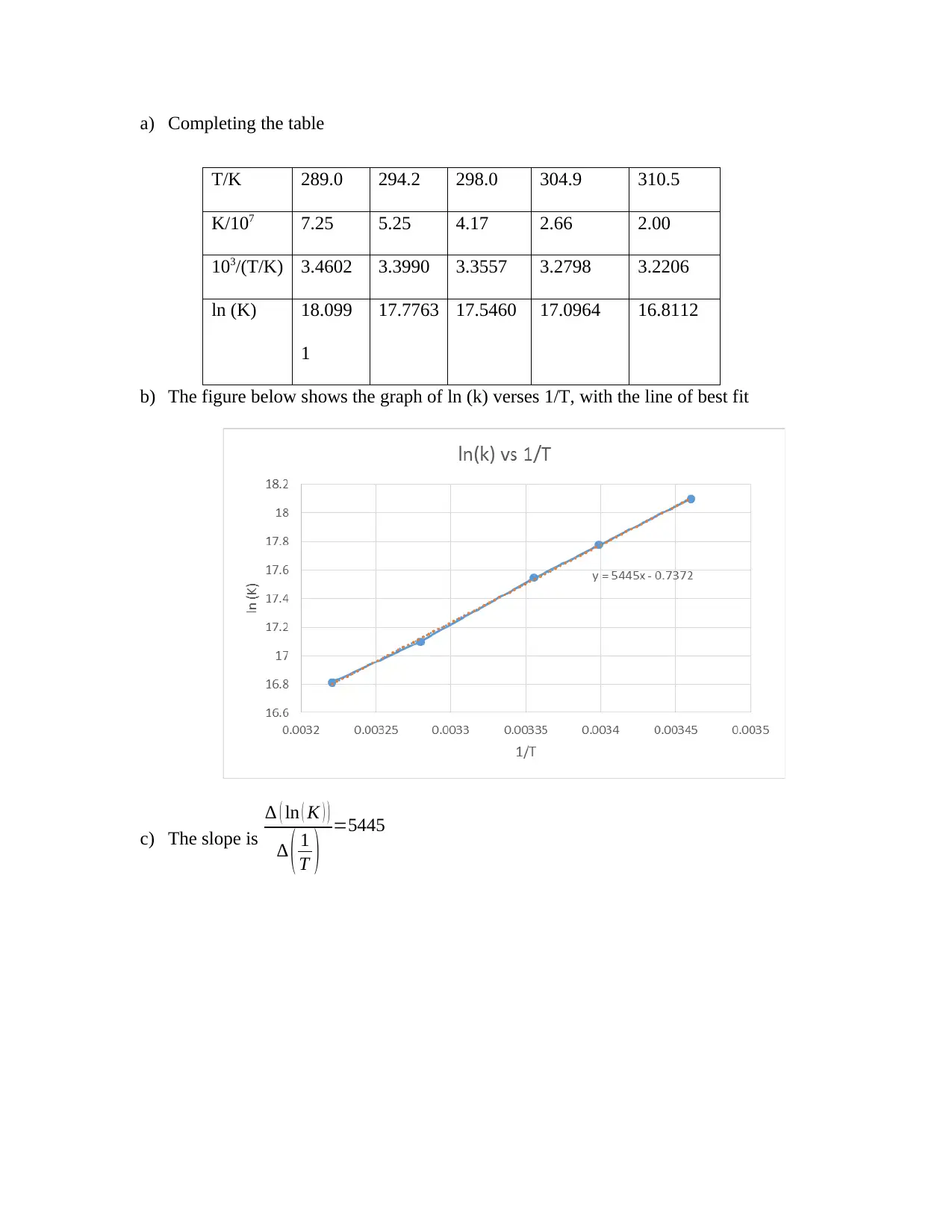
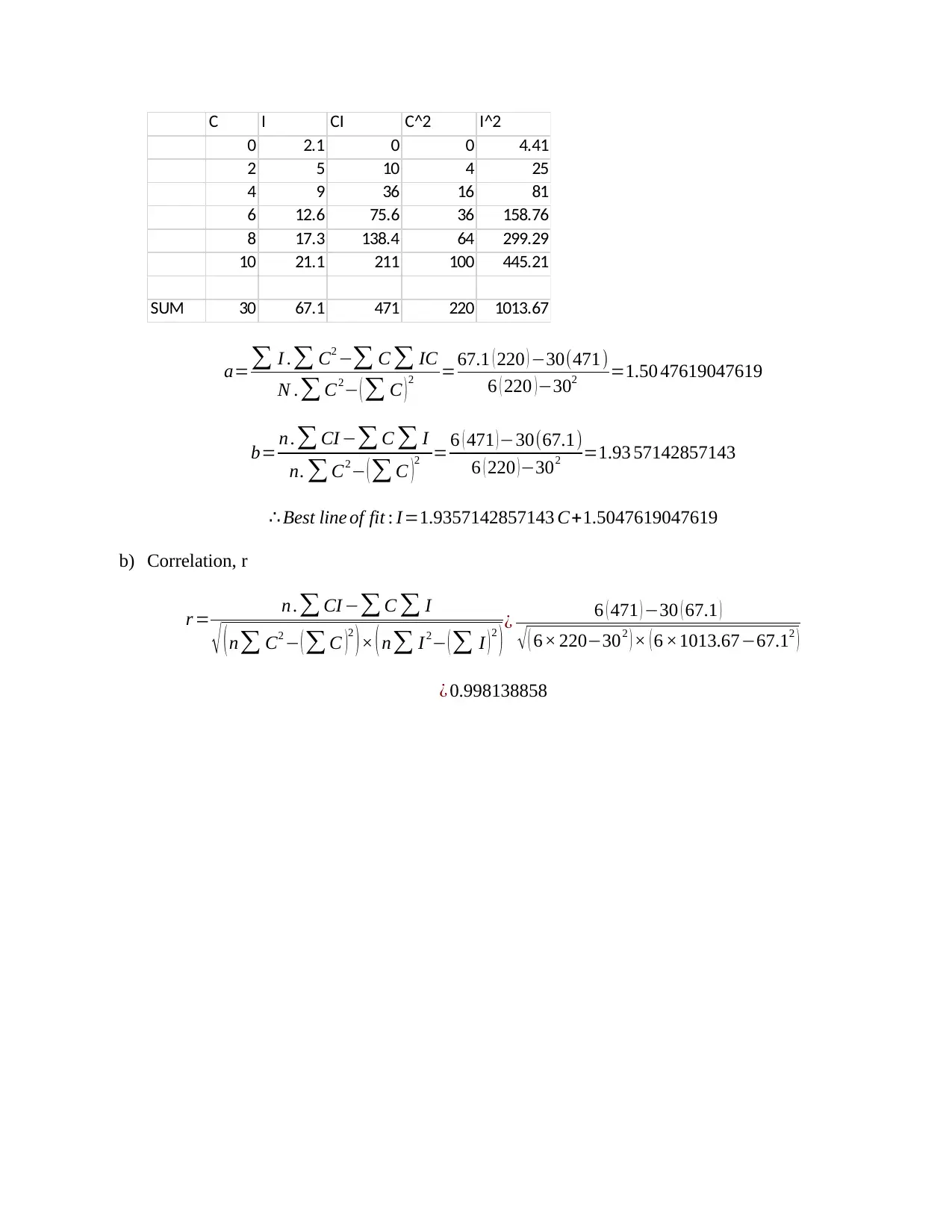
This chemistry assignment solution covers various aspects of chemical kinetics, equilibrium, and data analysis. It includes calculations related to first-order reactions, such as determining the equation for ln(c) and relating it to the form y=mx+c. The solution also demonstrates how to calculate the mass required to prepare a specific molarity solution of MgCl2 and how to determine the final concentration after diluting a NaOH solution. Furthermore, the assignment addresses the variation of the equilibrium constant with temperature, providing a table with temperature and equilibrium constant data, a graph of ln(K) versus 1/T, and the calculation of the slope. Additionally, the solution includes statistical analysis of enthalpy data, calculating the mean, median, range, standard deviation, and variance. It also covers the statistical analysis of arsenic concentration measurements, including the calculation of the mean, standard deviation, standard error of the mean, and the 95% confidence interval. Finally, the assignment includes problems on error propagation, such as calculating the total volume and average speed with associated uncertainties, and finding the best line of fit for fluorescence spectrometry data using linear regression and correlation analysis.
1 out of 5




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)