Risk Management in Drug Manufacturing: Process Analysis and Mitigation
VerifiedAdded on 2022/10/12
|16
|2867
|458
Report
AI Summary
This report delves into the critical aspects of risk management within the pharmaceutical manufacturing sector, specifically focusing on the production of a coated tablet for diabetes patients. It begins with a detailed process map outlining the stages of drug manufacturing from conceptualization to commercial launch. The report then analyzes potential failure modes, such as errors in raw material dispensing and compression, and their effects on product quality and patient safety. Risk assessment is performed using FMEA and FTA to determine the severity, occurrence, and detection of risks, along with calculating RPN values. The report proposes mitigation strategies and re-evaluates RPN values after mitigation. Furthermore, it addresses a specific risk question regarding the effective control strategy for a product launch, recommending HACCP and HAZOP as key tools. The analysis underscores the benefits and limitations of the analytical process, emphasizing the importance of proactive risk management to ensure product quality, regulatory compliance, and patient safety. The report concludes with a discussion of effective control strategies, including the use of HACCP and HAZOP, to address risks and ensure timely product launch, contributing to the success of the pharmaceutical manufacturing process.

Running head: RISK MANAGEMENT IN DRUG MANUFACTURING
RISK MANAGEMENT IN DRUG MANUFACTURING
Name of the Student
Name of the University
Author’s Note
RISK MANAGEMENT IN DRUG MANUFACTURING
Name of the Student
Name of the University
Author’s Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1RISK MANAGEMENT IN DRUG MANUFACTURING
Table of Contents
Introduction................................................................................................................................2
Discussion..................................................................................................................................2
PART 1.......................................................................................................................................2
1. Process map of the manufacturing process........................................................................2
2. Analysis of the manufacturing process..............................................................................5
a. Potential failure modes...................................................................................................5
b. Potential effect of the failure modes..............................................................................6
3. Risk assessment..................................................................................................................6
i. FMEA table construction................................................................................................6
ii. Severity, occurrence and detection scales......................................................................6
iii. RPN values...................................................................................................................8
iv. Mitigation for the risks..................................................................................................8
v. RPN values revisited after risk mitigation.....................................................................8
4. Benefits and limitations of this analytical process.............................................................9
PART 2:...................................................................................................................................10
1. Addressing risk question..................................................................................................10
2. Effective control strategy for the introduction of product X............................................10
Conclusion................................................................................................................................11
References................................................................................................................................12
Table of Contents
Introduction................................................................................................................................2
Discussion..................................................................................................................................2
PART 1.......................................................................................................................................2
1. Process map of the manufacturing process........................................................................2
2. Analysis of the manufacturing process..............................................................................5
a. Potential failure modes...................................................................................................5
b. Potential effect of the failure modes..............................................................................6
3. Risk assessment..................................................................................................................6
i. FMEA table construction................................................................................................6
ii. Severity, occurrence and detection scales......................................................................6
iii. RPN values...................................................................................................................8
iv. Mitigation for the risks..................................................................................................8
v. RPN values revisited after risk mitigation.....................................................................8
4. Benefits and limitations of this analytical process.............................................................9
PART 2:...................................................................................................................................10
1. Addressing risk question..................................................................................................10
2. Effective control strategy for the introduction of product X............................................10
Conclusion................................................................................................................................11
References................................................................................................................................12

2RISK MANAGEMENT IN DRUG MANUFACTURING
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3RISK MANAGEMENT IN DRUG MANUFACTURING
Introduction
The ABC Ltd. is a pharmaceutical manufacturer with a research and development unit
and a global manufacturing organization with research and development and manufacturing
sites worldwide. The key strategic priority of ABC ltd is to invest heavily in research and
development activities through research teams, mergers and partnerships and acquisition
contracts to develop and attain new molecular entity of pharmaceutical products which is
essential for the growth of the company. Besides the vast product assortment, the company
outsources several manufacturing tasks to contract manufacturing organizations (CMO) to
decrease costs while allowing the company to focus its attention on inventing, acquiring or
discovering new drug products and delivering the new products to the market.
The team at corporate headquarters has decided to transfer one of their products from
the USA to a contract manufacturing facility in Ireland. The product is a coated tablet for
diabetes patients. As an employee of the technology department of the contract
manufacturing company, the assignment requires evaluation of the product followed by
product assessment, product transfer processing and risk management required for the
transfer of the product.
Discussion
PART 1
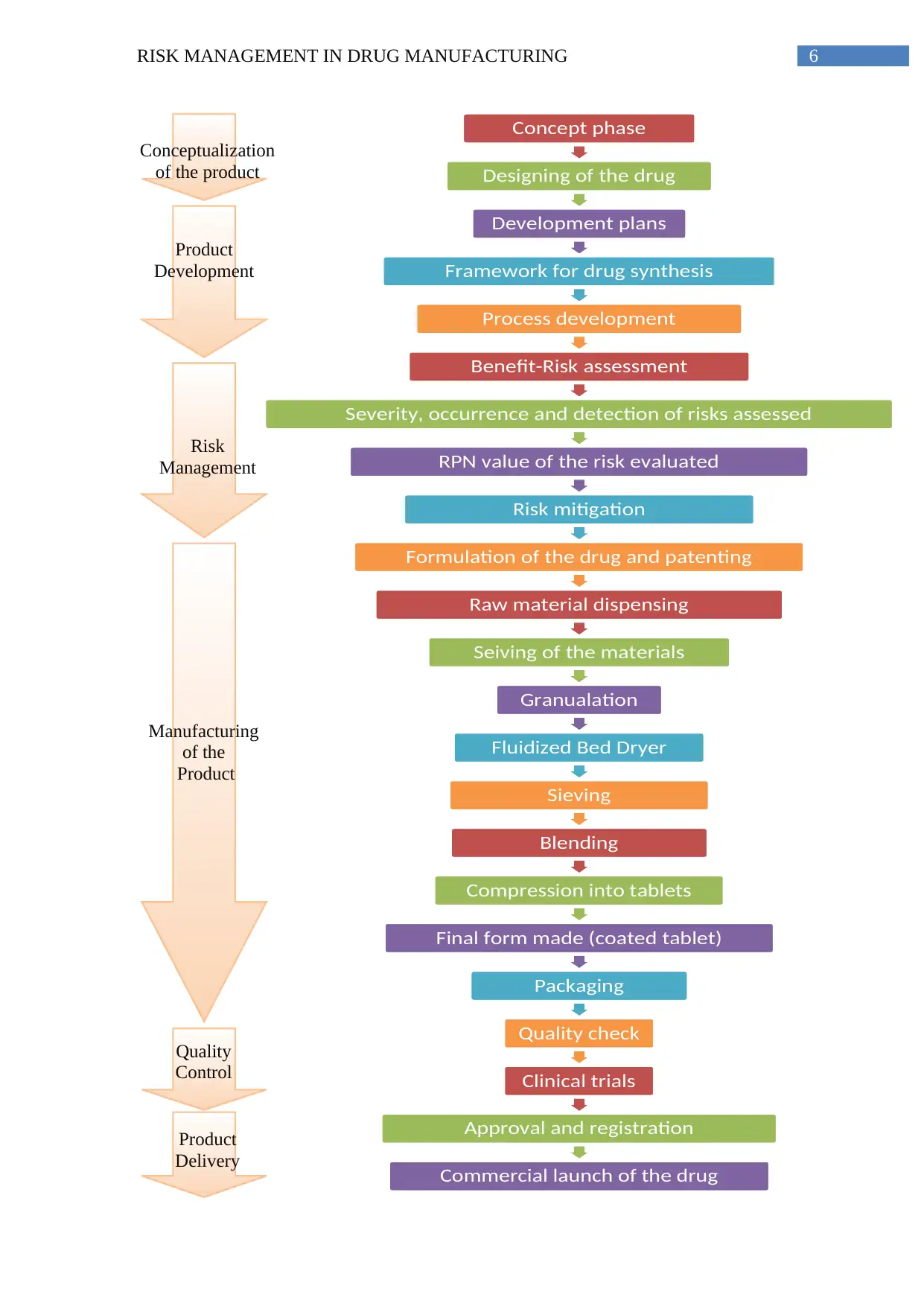
1. Process map of the manufacturing process
Manufacturing a product requires comprehensive conceptualization, followed by
careful planning and structuring of the development process, then safe and cautious
Introduction
The ABC Ltd. is a pharmaceutical manufacturer with a research and development unit
and a global manufacturing organization with research and development and manufacturing
sites worldwide. The key strategic priority of ABC ltd is to invest heavily in research and
development activities through research teams, mergers and partnerships and acquisition
contracts to develop and attain new molecular entity of pharmaceutical products which is
essential for the growth of the company. Besides the vast product assortment, the company
outsources several manufacturing tasks to contract manufacturing organizations (CMO) to
decrease costs while allowing the company to focus its attention on inventing, acquiring or
discovering new drug products and delivering the new products to the market.
The team at corporate headquarters has decided to transfer one of their products from
the USA to a contract manufacturing facility in Ireland. The product is a coated tablet for
diabetes patients. As an employee of the technology department of the contract
manufacturing company, the assignment requires evaluation of the product followed by
product assessment, product transfer processing and risk management required for the
transfer of the product.
Discussion
PART 1
1. Process map of the manufacturing process
Manufacturing a product requires comprehensive conceptualization, followed by
careful planning and structuring of the development process, then safe and cautious
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4RISK MANAGEMENT IN DRUG MANUFACTURING
manufacturing process, followed by critical quality checking and risk management of the
product, clinical trials, approval and legal documentation and finally the commercial launch
of the product.
Manufacturing and delivering a coated tablet for diabetes patients successfully
requires immense research backed conceptualization so as to deliver an innovative product
which addresses requirements that are not currently available in the market. Designing of the
drug must be evidence based and scientifically backed so that it concentrates on innovation,
cost effectiveness as well as feasibility of manufacturing the product. This is followed by
critical risk management which includes the potential risk and benefit of the drug followed by
cautious formulation of the medicine. Careful structuring of the developmental framework
and manufacturing procedure is essential before practical fabrication of the process (Damelio
2011).
Once the manufacturing starts, precautions must be taken to maintain a safe and
hazard free environment. The accuracy of the drug formulation and dosage must be
thoroughly checked before mass commercial production. The formulation must be patented to
reduce chances of industrial espionage. Accurate dispensing of the raw materials followed by
proper sieving, blending of the components to form tablets after adequate compression is
vital. After the final form of the drug is made which in this case is the coated tablet form, it
must be filled into casing properly. The waste generated must be disposed off carefully and
must follow the recommended government guidelines.
The manufacturing process must be followed by scrutinizing quality control process
which harbors comprehensive quality checking procedures followed by clinical trials. After
successful clinical trials, the product needs to be registered along with governmental approval
manufacturing process, followed by critical quality checking and risk management of the
product, clinical trials, approval and legal documentation and finally the commercial launch
of the product.
Manufacturing and delivering a coated tablet for diabetes patients successfully
requires immense research backed conceptualization so as to deliver an innovative product
which addresses requirements that are not currently available in the market. Designing of the
drug must be evidence based and scientifically backed so that it concentrates on innovation,
cost effectiveness as well as feasibility of manufacturing the product. This is followed by
critical risk management which includes the potential risk and benefit of the drug followed by
cautious formulation of the medicine. Careful structuring of the developmental framework
and manufacturing procedure is essential before practical fabrication of the process (Damelio
2011).
Once the manufacturing starts, precautions must be taken to maintain a safe and
hazard free environment. The accuracy of the drug formulation and dosage must be
thoroughly checked before mass commercial production. The formulation must be patented to
reduce chances of industrial espionage. Accurate dispensing of the raw materials followed by
proper sieving, blending of the components to form tablets after adequate compression is
vital. After the final form of the drug is made which in this case is the coated tablet form, it
must be filled into casing properly. The waste generated must be disposed off carefully and
must follow the recommended government guidelines.
The manufacturing process must be followed by scrutinizing quality control process
which harbors comprehensive quality checking procedures followed by clinical trials. After
successful clinical trials, the product needs to be registered along with governmental approval

5RISK MANAGEMENT IN DRUG MANUFACTURING
for commercialization. Then the product is ready for delivery following a commercial launch.
The following process map encompasses all the steps of the drug manufacturing procedure.
for commercialization. Then the product is ready for delivery following a commercial launch.
The following process map encompasses all the steps of the drug manufacturing procedure.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
6RISK MANAGEMENT IN DRUG MANUFACTURING
Concept phase
Designing of the drug
Development plans
Framework for drug synthesis
Process development
Benefit-Risk assessment
Severity, occurrence and detection of risks assessed
RPN value of the risk evaluated
Risk mitigation
Formulation of the drug and patenting
Raw material dispensing
Seiving of the materials
Granualation
Fluidized Bed Dryer
Sieving
Blending
Compression into tablets
Final form made (coated tablet)
Packaging
Quality check
Clinical trials
Approval and registration
Commercial launch of the drug
Conceptualization
of the product
Product
Development
Manufacturing
of the
Product
Quality
Control
Product
Delivery
Risk
Management
Concept phase
Designing of the drug
Development plans
Framework for drug synthesis
Process development
Benefit-Risk assessment
Severity, occurrence and detection of risks assessed
RPN value of the risk evaluated
Risk mitigation
Formulation of the drug and patenting
Raw material dispensing
Seiving of the materials
Granualation
Fluidized Bed Dryer
Sieving
Blending
Compression into tablets
Final form made (coated tablet)
Packaging
Quality check
Clinical trials
Approval and registration
Commercial launch of the drug
Conceptualization
of the product
Product
Development
Manufacturing
of the
Product
Quality
Control
Product
Delivery
Risk
Management
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7RISK MANAGEMENT IN DRUG MANUFACTURING
2. Analysis of the manufacturing process
a. Potential failure modes
Failure mode might be referred to as the source of a malfunction which might lead to the
failure or damage of the system. When a coordinated procedure has multiple potencies of
failing, it has various failure modes.
i. Dispensing of raw materials
Even with the correct drug designing and conceptualization, there might be
errors in drug formulation by inaccurate dispensing of raw materials which occurs
during industrial manufacturing of the drug. Incorrect dispensed quantity, wrong
material, cross contamination of the products and wrong labels might lead to incorrect
dispensing of raw materials. The raw materials must be thoroughly checked and the
protocols must be addressed accordingly to minimize the effects of such potential
failures.
ii. Compression procedure for tablet making
The errors associated with the dosage include flawed product selection,
inaccurate dose measurement along with considering the route of administration and
dose regimens. Use of incorrect temperature, segregation of the powder, incorrect
tooling, lack of trained personnel, incorrect speed of compression, faulty pH, errors in
amalgamation of the components and inaccuracy in the order of processing leading to
production of drug that is unstable, flawed and not usable. Compression errors might
compound other category of errors such as increased side effects, elevated risk factors
and decreased benefits of the drug.
2. Analysis of the manufacturing process
a. Potential failure modes
Failure mode might be referred to as the source of a malfunction which might lead to the
failure or damage of the system. When a coordinated procedure has multiple potencies of
failing, it has various failure modes.
i. Dispensing of raw materials
Even with the correct drug designing and conceptualization, there might be
errors in drug formulation by inaccurate dispensing of raw materials which occurs
during industrial manufacturing of the drug. Incorrect dispensed quantity, wrong
material, cross contamination of the products and wrong labels might lead to incorrect
dispensing of raw materials. The raw materials must be thoroughly checked and the
protocols must be addressed accordingly to minimize the effects of such potential
failures.
ii. Compression procedure for tablet making
The errors associated with the dosage include flawed product selection,
inaccurate dose measurement along with considering the route of administration and
dose regimens. Use of incorrect temperature, segregation of the powder, incorrect
tooling, lack of trained personnel, incorrect speed of compression, faulty pH, errors in
amalgamation of the components and inaccuracy in the order of processing leading to
production of drug that is unstable, flawed and not usable. Compression errors might
compound other category of errors such as increased side effects, elevated risk factors
and decreased benefits of the drug.

8RISK MANAGEMENT IN DRUG MANUFACTURING
b. Potential effect of the failure modes
Inaccurate dispensing of raw materials and inaccurate dosage along with faulty
compression during tablet manufacture if not checked and monitored will have a huge impact
on the company and if it makes to through commercialization, will give rise to grave
complications. Failure Modes and Effects Analysis (FMEA) is incorporated by organizations
as a tool for analyzing the causes of such failures and understanding the frequency and the
impact of such potential failure modes (Klochkov, Its and Vasilieva 2016).
3. Risk assessment
Critical analysis and statistical approaches must be used to maintain a robust set of
data and factual proofs. The data sets must be compared between different data sets to check
compatibility and reproducibility that is required during mass production of a product. Data
must be accessible, reliable and valid and backed by scientific evidences.
i. FMEA table construction
The FMEA table is attached with the assignment.
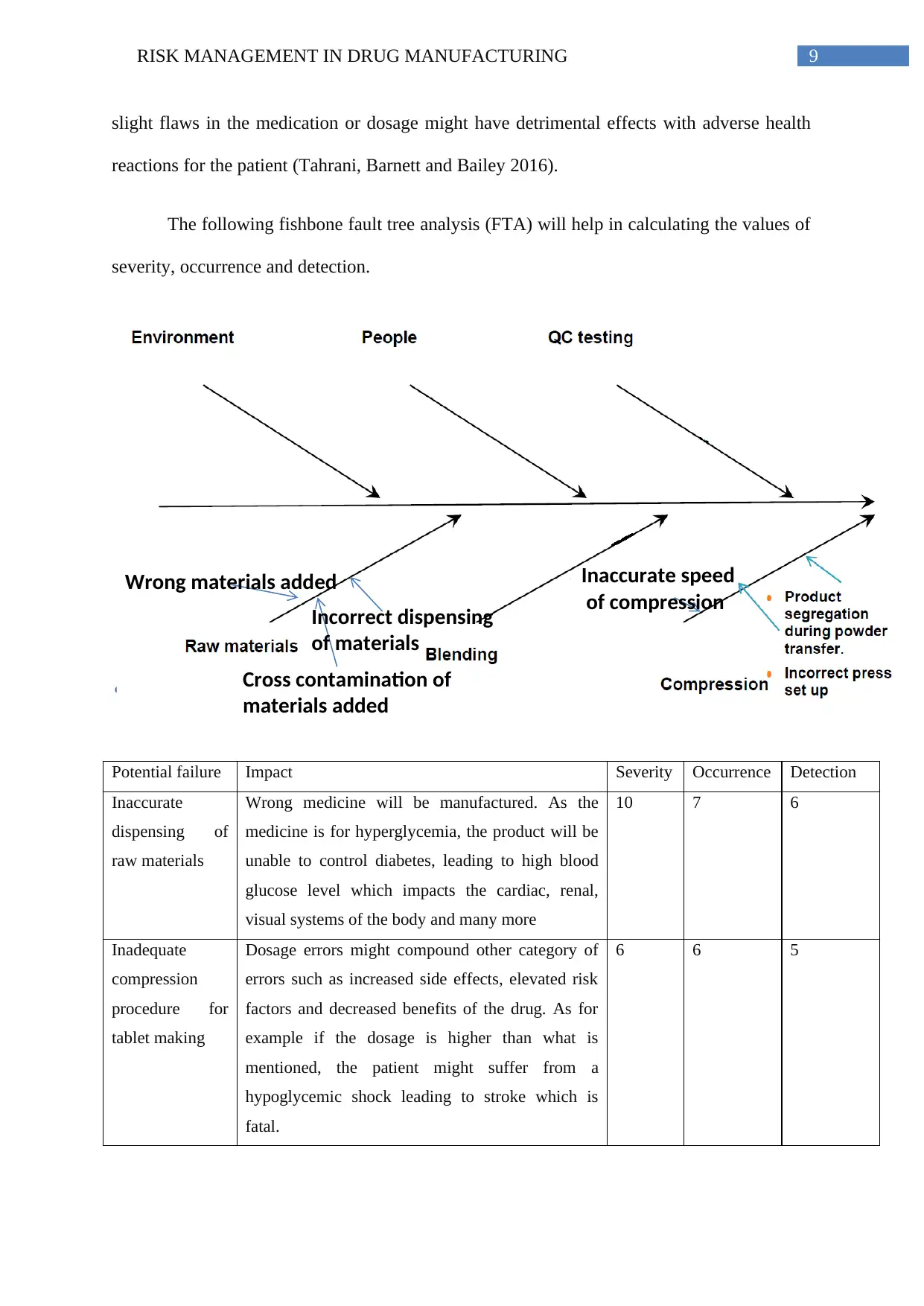
ii. Severity, occurrence and detection scales
The fault tree analysis (FTA) by the fishbone method helps in the determination of the
severity, occurrence and detection of the possible faults and probable failures of the
manufacturing process (Ishikawa and Lu 1985). Raw material dispensing stage is one of the
primary fundamental steps of drug manufacturing and incorrect quantities, or wrong
components or cross contamination between products might have devastating effects on the
final product and therefore is of maximum severity. Flawed compression protocol which is
lower on the fishbone structure of the FTA, will have lesser impact on the severity of the
failure however, as this is a pharmaceutical drug for diabetic hyperglycemic patients, even
b. Potential effect of the failure modes
Inaccurate dispensing of raw materials and inaccurate dosage along with faulty
compression during tablet manufacture if not checked and monitored will have a huge impact
on the company and if it makes to through commercialization, will give rise to grave
complications. Failure Modes and Effects Analysis (FMEA) is incorporated by organizations
as a tool for analyzing the causes of such failures and understanding the frequency and the
impact of such potential failure modes (Klochkov, Its and Vasilieva 2016).
3. Risk assessment
Critical analysis and statistical approaches must be used to maintain a robust set of
data and factual proofs. The data sets must be compared between different data sets to check
compatibility and reproducibility that is required during mass production of a product. Data
must be accessible, reliable and valid and backed by scientific evidences.
i. FMEA table construction
The FMEA table is attached with the assignment.
ii. Severity, occurrence and detection scales
The fault tree analysis (FTA) by the fishbone method helps in the determination of the
severity, occurrence and detection of the possible faults and probable failures of the
manufacturing process (Ishikawa and Lu 1985). Raw material dispensing stage is one of the
primary fundamental steps of drug manufacturing and incorrect quantities, or wrong
components or cross contamination between products might have devastating effects on the
final product and therefore is of maximum severity. Flawed compression protocol which is
lower on the fishbone structure of the FTA, will have lesser impact on the severity of the
failure however, as this is a pharmaceutical drug for diabetic hyperglycemic patients, even
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
9RISK MANAGEMENT IN DRUG MANUFACTURING
slight flaws in the medication or dosage might have detrimental effects with adverse health
reactions for the patient (Tahrani, Barnett and Bailey 2016).
The following fishbone fault tree analysis (FTA) will help in calculating the values of
severity, occurrence and detection.
Potential failure Impact Severity Occurrence Detection
Inaccurate
dispensing of
raw materials
Wrong medicine will be manufactured. As the
medicine is for hyperglycemia, the product will be
unable to control diabetes, leading to high blood
glucose level which impacts the cardiac, renal,
visual systems of the body and many more
10 7 6
Inadequate
compression
procedure for
tablet making
Dosage errors might compound other category of
errors such as increased side effects, elevated risk
factors and decreased benefits of the drug. As for
example if the dosage is higher than what is
mentioned, the patient might suffer from a
hypoglycemic shock leading to stroke which is
fatal.
6 6 5
Inaccurate speed
of compression
Wrong materials added
Cross contamination of
materials added
Incorrect dispensing
of materials
slight flaws in the medication or dosage might have detrimental effects with adverse health
reactions for the patient (Tahrani, Barnett and Bailey 2016).
The following fishbone fault tree analysis (FTA) will help in calculating the values of
severity, occurrence and detection.
Potential failure Impact Severity Occurrence Detection
Inaccurate
dispensing of
raw materials
Wrong medicine will be manufactured. As the
medicine is for hyperglycemia, the product will be
unable to control diabetes, leading to high blood
glucose level which impacts the cardiac, renal,
visual systems of the body and many more
10 7 6
Inadequate
compression
procedure for
tablet making
Dosage errors might compound other category of
errors such as increased side effects, elevated risk
factors and decreased benefits of the drug. As for
example if the dosage is higher than what is
mentioned, the patient might suffer from a
hypoglycemic shock leading to stroke which is
fatal.
6 6 5
Inaccurate speed
of compression
Wrong materials added
Cross contamination of
materials added
Incorrect dispensing
of materials
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
10RISK MANAGEMENT IN DRUG MANUFACTURING
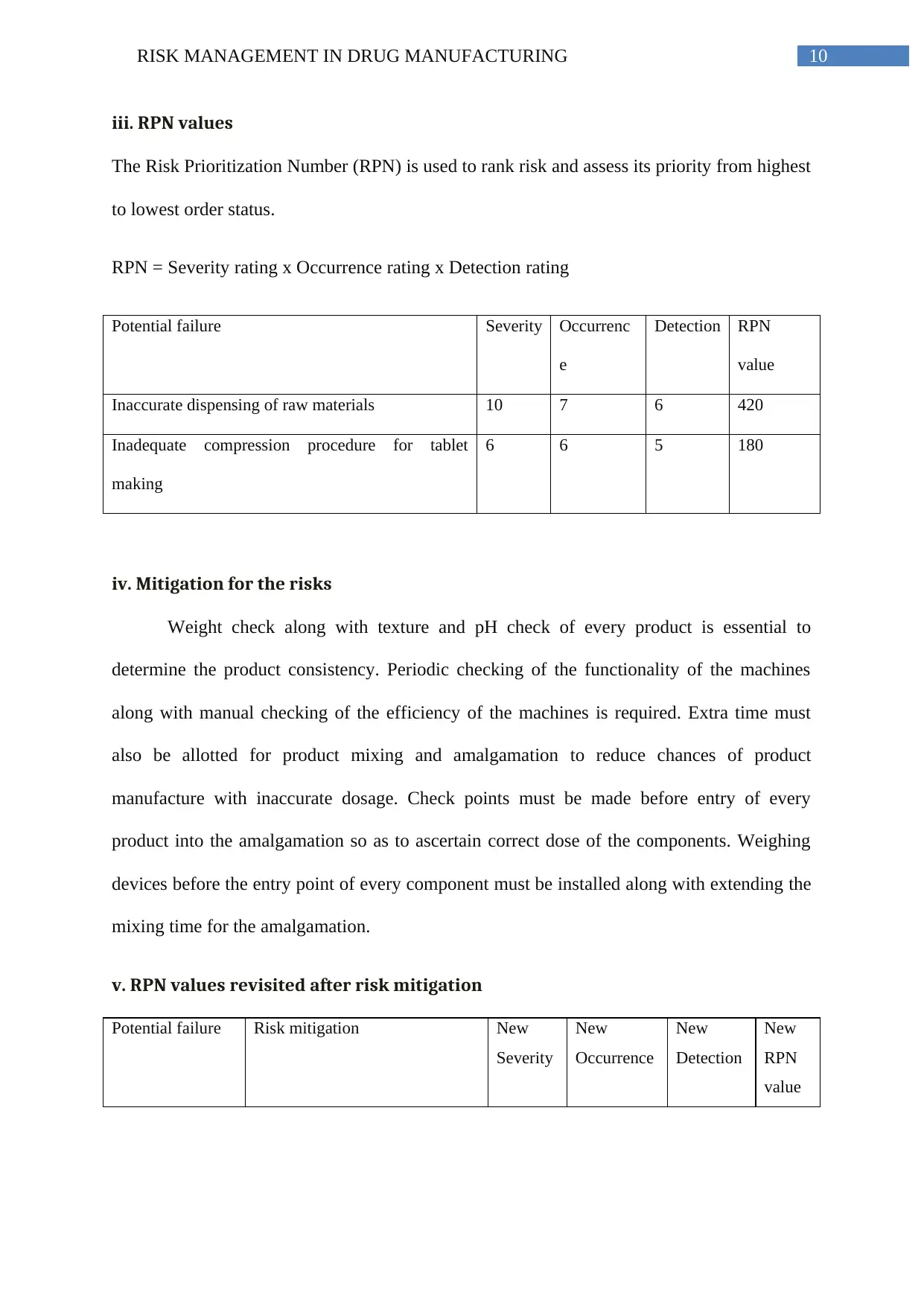
iii. RPN values
The Risk Prioritization Number (RPN) is used to rank risk and assess its priority from highest
to lowest order status.
RPN = Severity rating x Occurrence rating x Detection rating
Potential failure Severity Occurrenc
e
Detection RPN
value
Inaccurate dispensing of raw materials 10 7 6 420
Inadequate compression procedure for tablet
making
6 6 5 180
iv. Mitigation for the risks
Weight check along with texture and pH check of every product is essential to
determine the product consistency. Periodic checking of the functionality of the machines
along with manual checking of the efficiency of the machines is required. Extra time must
also be allotted for product mixing and amalgamation to reduce chances of product
manufacture with inaccurate dosage. Check points must be made before entry of every
product into the amalgamation so as to ascertain correct dose of the components. Weighing
devices before the entry point of every component must be installed along with extending the
mixing time for the amalgamation.
v. RPN values revisited after risk mitigation
Potential failure Risk mitigation New
Severity
New
Occurrence
New
Detection
New
RPN
value
iii. RPN values
The Risk Prioritization Number (RPN) is used to rank risk and assess its priority from highest
to lowest order status.
RPN = Severity rating x Occurrence rating x Detection rating
Potential failure Severity Occurrenc
e
Detection RPN
value
Inaccurate dispensing of raw materials 10 7 6 420
Inadequate compression procedure for tablet
making
6 6 5 180
iv. Mitigation for the risks
Weight check along with texture and pH check of every product is essential to
determine the product consistency. Periodic checking of the functionality of the machines
along with manual checking of the efficiency of the machines is required. Extra time must
also be allotted for product mixing and amalgamation to reduce chances of product
manufacture with inaccurate dosage. Check points must be made before entry of every
product into the amalgamation so as to ascertain correct dose of the components. Weighing
devices before the entry point of every component must be installed along with extending the
mixing time for the amalgamation.
v. RPN values revisited after risk mitigation
Potential failure Risk mitigation New
Severity
New
Occurrence
New
Detection
New
RPN
value
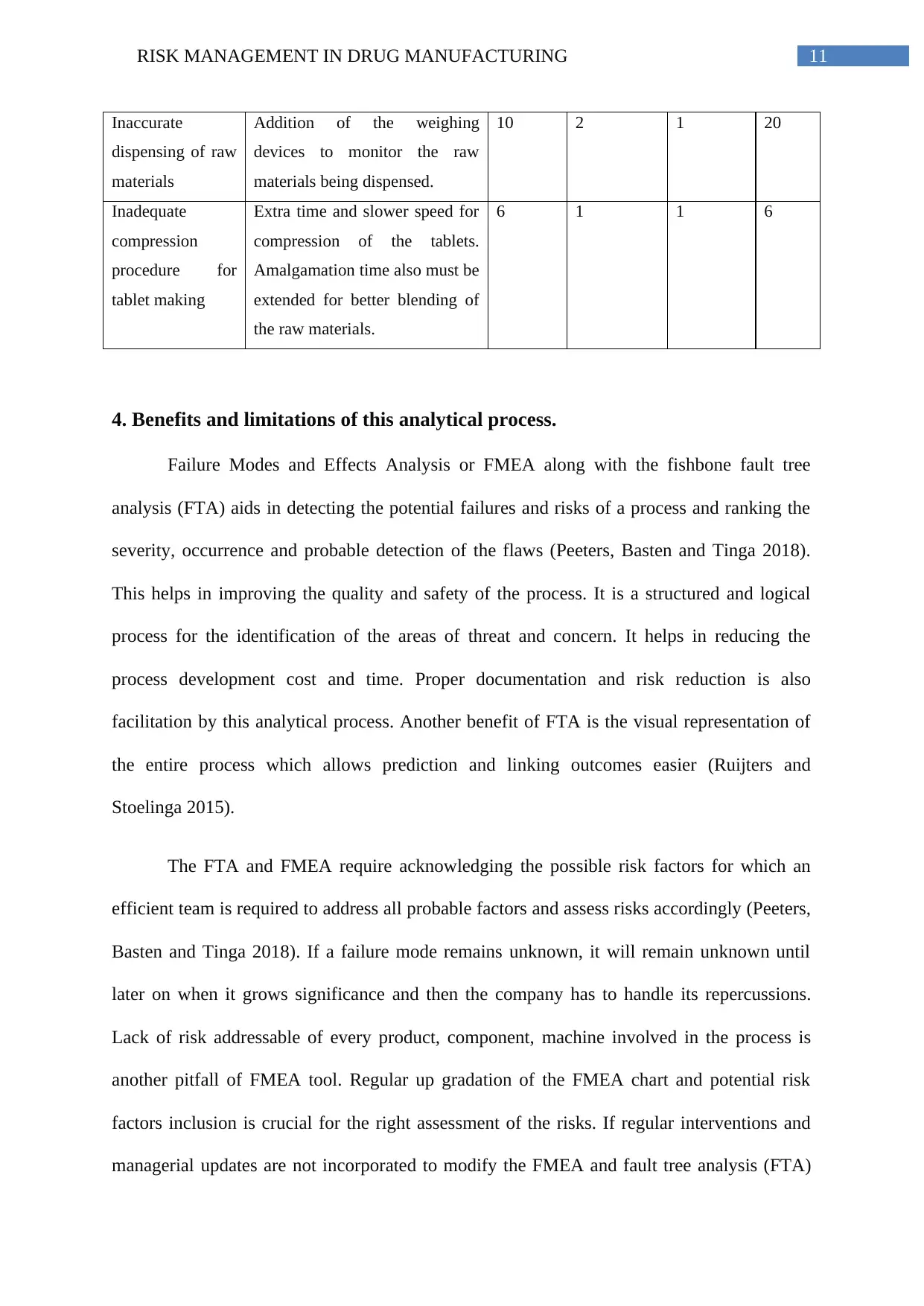
11RISK MANAGEMENT IN DRUG MANUFACTURING
Inaccurate
dispensing of raw
materials
Addition of the weighing
devices to monitor the raw
materials being dispensed.
10 2 1 20
Inadequate
compression
procedure for
tablet making
Extra time and slower speed for
compression of the tablets.
Amalgamation time also must be
extended for better blending of
the raw materials.
6 1 1 6
4. Benefits and limitations of this analytical process.
Failure Modes and Effects Analysis or FMEA along with the fishbone fault tree
analysis (FTA) aids in detecting the potential failures and risks of a process and ranking the
severity, occurrence and probable detection of the flaws (Peeters, Basten and Tinga 2018).
This helps in improving the quality and safety of the process. It is a structured and logical
process for the identification of the areas of threat and concern. It helps in reducing the
process development cost and time. Proper documentation and risk reduction is also
facilitation by this analytical process. Another benefit of FTA is the visual representation of
the entire process which allows prediction and linking outcomes easier (Ruijters and
Stoelinga 2015).
The FTA and FMEA require acknowledging the possible risk factors for which an
efficient team is required to address all probable factors and assess risks accordingly (Peeters,
Basten and Tinga 2018). If a failure mode remains unknown, it will remain unknown until
later on when it grows significance and then the company has to handle its repercussions.
Lack of risk addressable of every product, component, machine involved in the process is
another pitfall of FMEA tool. Regular up gradation of the FMEA chart and potential risk
factors inclusion is crucial for the right assessment of the risks. If regular interventions and
managerial updates are not incorporated to modify the FMEA and fault tree analysis (FTA)
Inaccurate
dispensing of raw
materials
Addition of the weighing
devices to monitor the raw
materials being dispensed.
10 2 1 20
Inadequate
compression
procedure for
tablet making
Extra time and slower speed for
compression of the tablets.
Amalgamation time also must be
extended for better blending of
the raw materials.
6 1 1 6
4. Benefits and limitations of this analytical process.
Failure Modes and Effects Analysis or FMEA along with the fishbone fault tree
analysis (FTA) aids in detecting the potential failures and risks of a process and ranking the
severity, occurrence and probable detection of the flaws (Peeters, Basten and Tinga 2018).
This helps in improving the quality and safety of the process. It is a structured and logical
process for the identification of the areas of threat and concern. It helps in reducing the
process development cost and time. Proper documentation and risk reduction is also
facilitation by this analytical process. Another benefit of FTA is the visual representation of
the entire process which allows prediction and linking outcomes easier (Ruijters and
Stoelinga 2015).
The FTA and FMEA require acknowledging the possible risk factors for which an
efficient team is required to address all probable factors and assess risks accordingly (Peeters,
Basten and Tinga 2018). If a failure mode remains unknown, it will remain unknown until
later on when it grows significance and then the company has to handle its repercussions.
Lack of risk addressable of every product, component, machine involved in the process is
another pitfall of FMEA tool. Regular up gradation of the FMEA chart and potential risk
factors inclusion is crucial for the right assessment of the risks. If regular interventions and
managerial updates are not incorporated to modify the FMEA and fault tree analysis (FTA)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



