ABC Hospital: Risk Assessment, Quality, and PDCA Cycle Analysis
VerifiedAdded on 2023/06/10
|10
|1940
|256
Report
AI Summary
This report provides a critical analysis of ABC Hospital's approach to risk assessment and quality management, focusing on the safe disposal of medical waste. The study utilizes the PDCA cycle (Plan, Do, Check, Act) to devise and evaluate quality improvement strategies. The 'Plan' phase involves identifying existing waste treatment options and formulating objectives. The 'Do' phase includes researching best practices and assessing the budget for new equipment. The 'Check' phase involves verifying the efficacy of installed devices and assessing contamination reduction. The 'Act' phase analyzes actions and efficiency using defined parameters, contrasting outcomes with past data. The report proposes the installation of an automatic autoclave, medical waste incinerator, vitrification chambers, and irradiation devices, along with staff training. The conclusion emphasizes the importance of quality management in healthcare, particularly in managing hazardous biomedical waste, and highlights the effectiveness of the proposed strategies based on the analysis of community feedback and data comparison, emphasizing the need for continuous improvement and strict supervision.

Running head: RISK ASSESSMENT AND QUALITY IN HEALTH
RISK ASSESSMENT AND QUALITY IN HEALTH
Name of the Student:
Name of the University:
Author Note:
RISK ASSESSMENT AND QUALITY IN HEALTH
Name of the Student:
Name of the University:
Author Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1RISK ASSESSMENT AND QUALITY IN HEALTH
Introduction
Healthcare organizations strive to provide the best of medical facilities to the patients
all over the world. In order to ensure proper disposal of the facilities, it is extremely
important to maintain a frequent quality check and ensure that the services being dispensed
are up to the mark (Bernardo et al.2015). This assignment deals with the critical analysis of
‘ABC hospital’ and devise an innovation in terms of quality improvement using the PDCA
cycle.
Background:
The ‘ABC’ hospital is one of the most renowned hospitals that has an excellent group
of doctors working for the organization. The hospital has a strict hierarchal structure and
operations are carried out in accordance of the same classification. The first level of hierarchy
is occupied by the administrative post holders such as the board of directors headed by the
CEO. Under the first classification is a segmentation of various portfolios dealing with
business operation, emergency care, ancillary services and customer support. The hospital has
an efficient quality control unit as well that is responsible for monitoring and double
verifying the pharmaceutical products being used at the hospital (Clark et al.2013). However,
it has been observed that despite maintaining the quality check regularly there are some
unidentified lacunas that are not addressed adequately with the parameters of quality control
standards. The identified quality concern with respect to ‘ABC hospital’ is the problem
associated with the safe disposal of medical wastes to minimise contamination or
transmission of infections.
Introduction
Healthcare organizations strive to provide the best of medical facilities to the patients
all over the world. In order to ensure proper disposal of the facilities, it is extremely
important to maintain a frequent quality check and ensure that the services being dispensed
are up to the mark (Bernardo et al.2015). This assignment deals with the critical analysis of
‘ABC hospital’ and devise an innovation in terms of quality improvement using the PDCA
cycle.
Background:
The ‘ABC’ hospital is one of the most renowned hospitals that has an excellent group
of doctors working for the organization. The hospital has a strict hierarchal structure and
operations are carried out in accordance of the same classification. The first level of hierarchy
is occupied by the administrative post holders such as the board of directors headed by the
CEO. Under the first classification is a segmentation of various portfolios dealing with
business operation, emergency care, ancillary services and customer support. The hospital has
an efficient quality control unit as well that is responsible for monitoring and double
verifying the pharmaceutical products being used at the hospital (Clark et al.2013). However,
it has been observed that despite maintaining the quality check regularly there are some
unidentified lacunas that are not addressed adequately with the parameters of quality control
standards. The identified quality concern with respect to ‘ABC hospital’ is the problem
associated with the safe disposal of medical wastes to minimise contamination or
transmission of infections.

2RISK ASSESSMENT AND QUALITY IN HEALTH
Application of PDCA cycle:
Fig: The PDCA Cycle
The PDCA cycle or the Plan, Do, Check and Act cycle is the most famous business
tool that is used to check and compare the efficiency of a new business strategy that is
expected to be introduced for the expand the profit margins of the company (De Oliveira
2013). However, it is critical here to note here that the PDCA cycle is not restricted to be
used only as a business tool that would take care of the innovation in terms of business
strategies. The PDCA cycle in this assignment has been used to devise a new approach to
deal with the problems associated with the safe disposal of biological waste and hence it can
be commented that in this context the cycle has been used to study the effectiveness of a new
quality assurance strategy (Johnson 2016). The following sections would deal with the
Do Plan
ActCheck
Application of PDCA cycle:
Fig: The PDCA Cycle
The PDCA cycle or the Plan, Do, Check and Act cycle is the most famous business
tool that is used to check and compare the efficiency of a new business strategy that is
expected to be introduced for the expand the profit margins of the company (De Oliveira
2013). However, it is critical here to note here that the PDCA cycle is not restricted to be
used only as a business tool that would take care of the innovation in terms of business
strategies. The PDCA cycle in this assignment has been used to devise a new approach to
deal with the problems associated with the safe disposal of biological waste and hence it can
be commented that in this context the cycle has been used to study the effectiveness of a new
quality assurance strategy (Johnson 2016). The following sections would deal with the
Do Plan
ActCheck
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3RISK ASSESSMENT AND QUALITY IN HEALTH
considerations of factors listed under each of the segments of the PDCA cycle. Each of the
factors are critically considered before the implementation of the new strategy (Matsuo and
Nakahara 2013).
Plan:
Planning involves the thought process that is responsible behind the designing of the
new approach. It involves keeping in mind a set of ethical considerations and answering a set
of framed questions in order to assert the objective related to the fulfilment of the expected
outcome. In this case, the concern is regarding the safe disposal of the hazardous biological
waste and the concerned questions would include the following,
What are the existing waste treatment options in the hospital?
To what extent is the designing of a new approach required?
How would the effectiveness of the implemented new strategy accessed?
Based on these questions the planning objectives of the quality project that has been
undertaken is asserted.
Do:
This segment identifies the list of actions that need to be undertaken in order to satisfy
the above stated objectives. It involves conducting a thorough research on the best disposal
techniques used by other hospitals. It also deals with the comparison of the hospital budget in
order to ensure the possibilities of incorporating newer machines that can successfully treat
the medical waste generated at the hospital and reduce the contaminations associated with it
(Peitrzak and Paliszkiewicz 2015). This segment deals with the preparation and assessment of
available budget for the successful implementation of the new innovations.
considerations of factors listed under each of the segments of the PDCA cycle. Each of the
factors are critically considered before the implementation of the new strategy (Matsuo and
Nakahara 2013).
Plan:
Planning involves the thought process that is responsible behind the designing of the
new approach. It involves keeping in mind a set of ethical considerations and answering a set
of framed questions in order to assert the objective related to the fulfilment of the expected
outcome. In this case, the concern is regarding the safe disposal of the hazardous biological
waste and the concerned questions would include the following,
What are the existing waste treatment options in the hospital?
To what extent is the designing of a new approach required?
How would the effectiveness of the implemented new strategy accessed?
Based on these questions the planning objectives of the quality project that has been
undertaken is asserted.
Do:
This segment identifies the list of actions that need to be undertaken in order to satisfy
the above stated objectives. It involves conducting a thorough research on the best disposal
techniques used by other hospitals. It also deals with the comparison of the hospital budget in
order to ensure the possibilities of incorporating newer machines that can successfully treat
the medical waste generated at the hospital and reduce the contaminations associated with it
(Peitrzak and Paliszkiewicz 2015). This segment deals with the preparation and assessment of
available budget for the successful implementation of the new innovations.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4RISK ASSESSMENT AND QUALITY IN HEALTH
Check:
This segment deals with double verification of the installed devices that are
incorporated for the efficient management of waste disposal. The first phase of this segment
involves performing a thorough scrutiny on the efficacy of the current installed devices and
the second phase deals with accessing the reduction rate of contamination in the hazardous
sample in comparison to the data recorded earlier before the successful implementation of the
treatment devices (Runciman et al.2017).
Act:
This segment involves the analysis of the series of actions that have been undertaken
and accessing the degree of enhanced efficiency using a set of defined parameters. In case of
‘ABC hospital’ the effectiveness of actions undertaken are considered according to the
databases recorded in the past. The possibility of expecting a positive outcome is contrasted
with the achieved outcomes and in the same way the further strategies involving the same
principles are devised (Salman et al.2014).
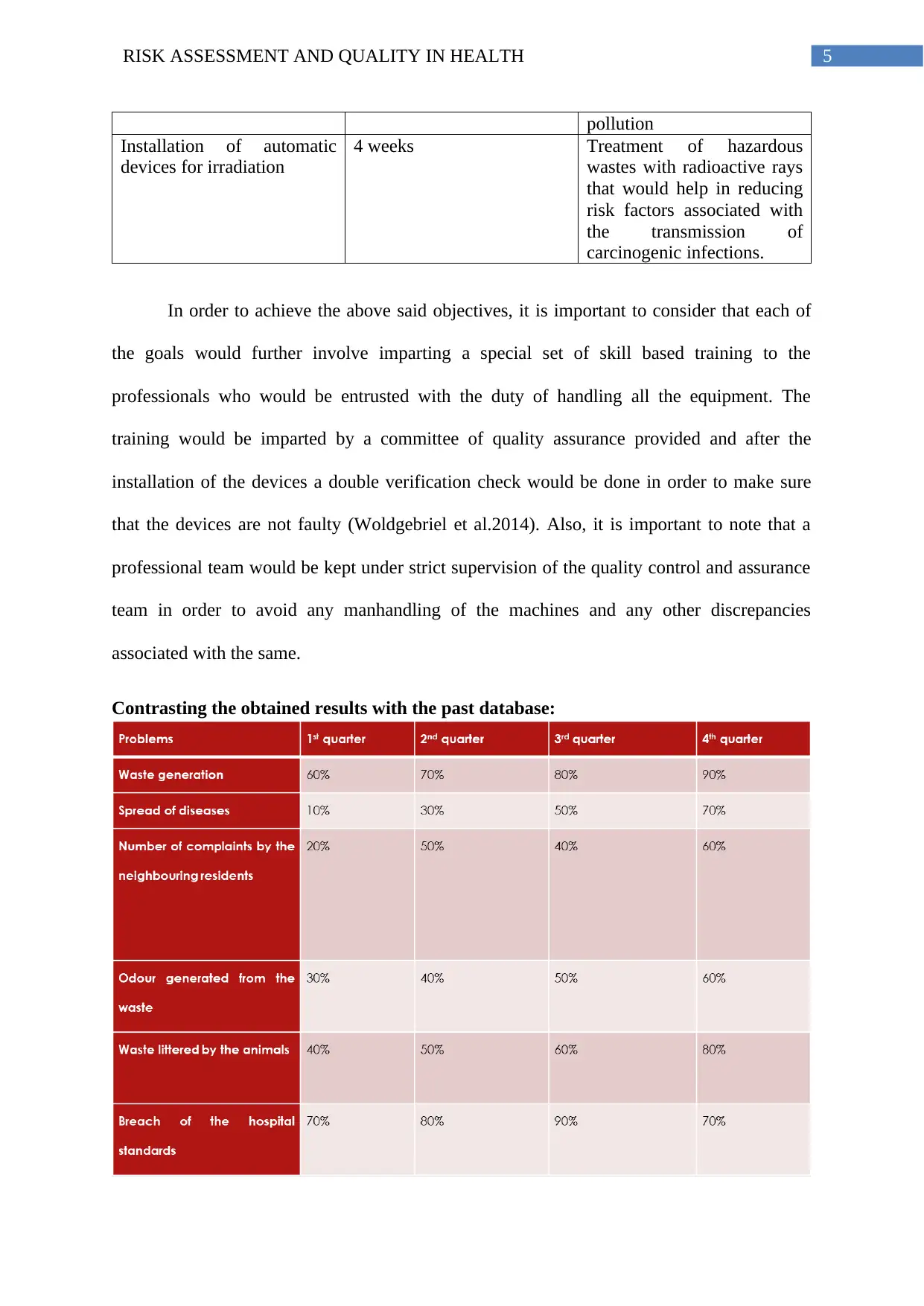
The following table shows the necessary actions that need to be undertaken in order to
efficiently incorporate the change related to quality consideration so that the efficacy of the
project can be improved.
Quality-improvement
innovation
Requisite time span Expected outcome
Installation of in-house
automatic autoclave
machine
1-2 weeks Complete sterilisation of
bacterial wastes including
cessation of bacterial spores
Installation of a medical
waste incinerator
1-3 weeks Efficient and controlled
burning down of infectious
medical wastes in order to
reduce risks associated with
contamination
Installation of Vitrification
chambers
3-5 weeks Conversion of high risk
associated medical
hazardous wastes to glass to
avoid problems related to
diffusion and environmental
Check:
This segment deals with double verification of the installed devices that are
incorporated for the efficient management of waste disposal. The first phase of this segment
involves performing a thorough scrutiny on the efficacy of the current installed devices and
the second phase deals with accessing the reduction rate of contamination in the hazardous
sample in comparison to the data recorded earlier before the successful implementation of the
treatment devices (Runciman et al.2017).
Act:
This segment involves the analysis of the series of actions that have been undertaken
and accessing the degree of enhanced efficiency using a set of defined parameters. In case of
‘ABC hospital’ the effectiveness of actions undertaken are considered according to the
databases recorded in the past. The possibility of expecting a positive outcome is contrasted
with the achieved outcomes and in the same way the further strategies involving the same
principles are devised (Salman et al.2014).
The following table shows the necessary actions that need to be undertaken in order to
efficiently incorporate the change related to quality consideration so that the efficacy of the
project can be improved.
Quality-improvement
innovation
Requisite time span Expected outcome
Installation of in-house
automatic autoclave
machine
1-2 weeks Complete sterilisation of
bacterial wastes including
cessation of bacterial spores
Installation of a medical
waste incinerator
1-3 weeks Efficient and controlled
burning down of infectious
medical wastes in order to
reduce risks associated with
contamination
Installation of Vitrification
chambers
3-5 weeks Conversion of high risk
associated medical
hazardous wastes to glass to
avoid problems related to
diffusion and environmental
5RISK ASSESSMENT AND QUALITY IN HEALTH
pollution
Installation of automatic
devices for irradiation
4 weeks Treatment of hazardous
wastes with radioactive rays
that would help in reducing
risk factors associated with
the transmission of
carcinogenic infections.
In order to achieve the above said objectives, it is important to consider that each of
the goals would further involve imparting a special set of skill based training to the
professionals who would be entrusted with the duty of handling all the equipment. The
training would be imparted by a committee of quality assurance provided and after the
installation of the devices a double verification check would be done in order to make sure
that the devices are not faulty (Woldgebriel et al.2014). Also, it is important to note that a
professional team would be kept under strict supervision of the quality control and assurance
team in order to avoid any manhandling of the machines and any other discrepancies
associated with the same.
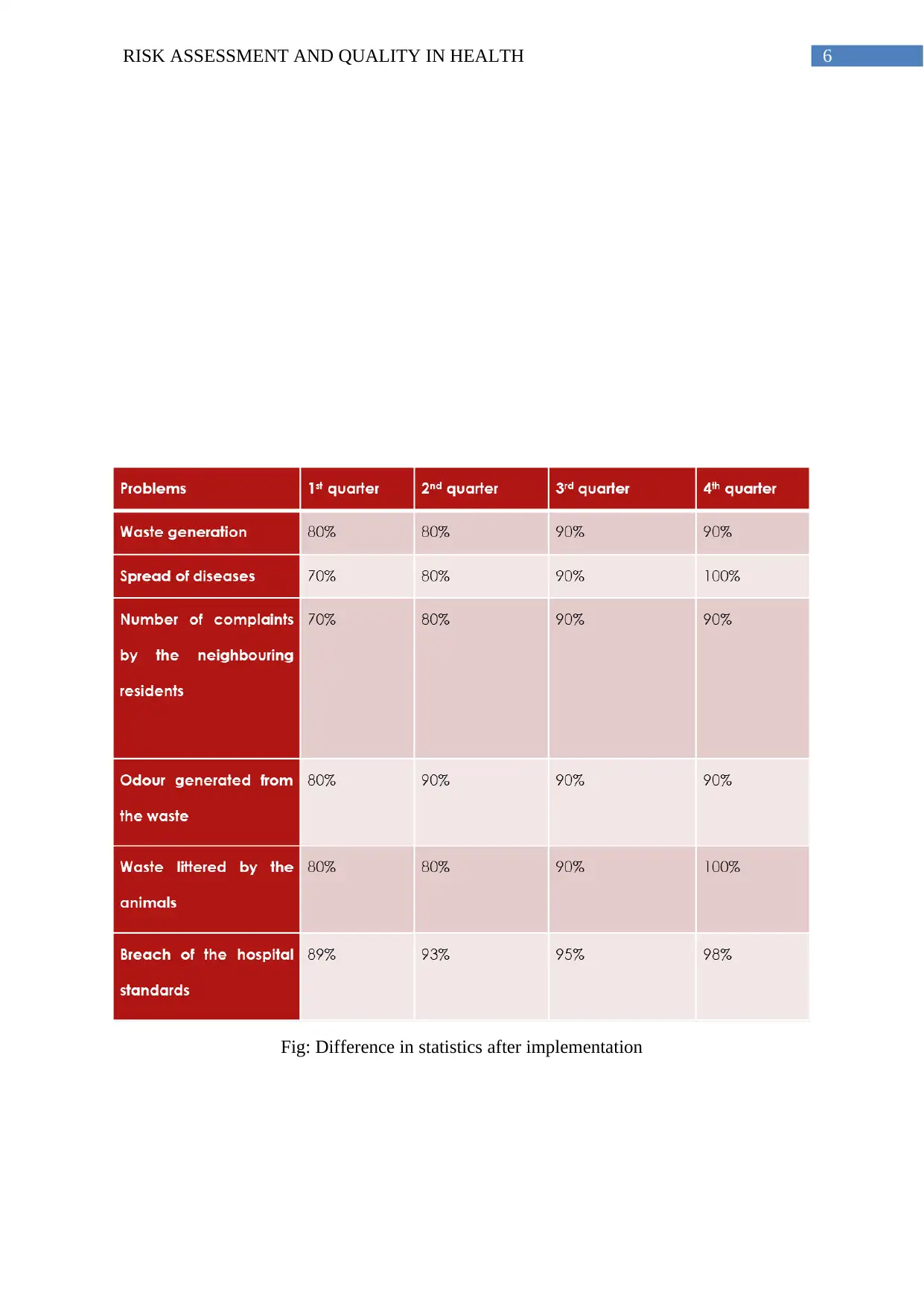
Contrasting the obtained results with the past database:
pollution
Installation of automatic
devices for irradiation
4 weeks Treatment of hazardous
wastes with radioactive rays
that would help in reducing
risk factors associated with
the transmission of
carcinogenic infections.
In order to achieve the above said objectives, it is important to consider that each of
the goals would further involve imparting a special set of skill based training to the
professionals who would be entrusted with the duty of handling all the equipment. The
training would be imparted by a committee of quality assurance provided and after the
installation of the devices a double verification check would be done in order to make sure
that the devices are not faulty (Woldgebriel et al.2014). Also, it is important to note that a
professional team would be kept under strict supervision of the quality control and assurance
team in order to avoid any manhandling of the machines and any other discrepancies
associated with the same.
Contrasting the obtained results with the past database:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
6RISK ASSESSMENT AND QUALITY IN HEALTH
Fig: Difference in statistics after implementation
Fig: Difference in statistics after implementation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7RISK ASSESSMENT AND QUALITY IN HEALTH
Conclusion:
Quality management is an integral part of hospital administration and it is extremely
important to note that the quality assurance is not a parameter to compromise. The paper
deals with the management of hazardous biomedical waste and it is one of the most critical
considerations related to hospital management industry as it leads to the transmission of a
huge number of infectious diseases and deterioration of community health and hygiene. The
paper has primary highlighted the major issues pertaining to the problems associated with bio
medical waste treatment in close association with the ‘ABC hospital’. Further, the paper also
focuses on the quality improvement strategies that have been designed in order to be
undertaken by the concerned hospital. The strategies include the use of biomedical treatment
devices such as incinerators, autoclave machines, vitrification and irradiation chambers. The
implementation of the strategies include considering each of the four aspects of the PDCA
cycle at each and every step. Also, in order to access the success rate of the implemented
strategies a comparison with respect to the database of the previous year was analysed based
on the criteria of complains from the community based near the hospital in terms of
percentage of infection, foul odour, breach of hospital policies, spreading of litter by local
animals and number of complains. The results showed a significant decrease in all the above
Conclusion:
Quality management is an integral part of hospital administration and it is extremely
important to note that the quality assurance is not a parameter to compromise. The paper
deals with the management of hazardous biomedical waste and it is one of the most critical
considerations related to hospital management industry as it leads to the transmission of a
huge number of infectious diseases and deterioration of community health and hygiene. The
paper has primary highlighted the major issues pertaining to the problems associated with bio
medical waste treatment in close association with the ‘ABC hospital’. Further, the paper also
focuses on the quality improvement strategies that have been designed in order to be
undertaken by the concerned hospital. The strategies include the use of biomedical treatment
devices such as incinerators, autoclave machines, vitrification and irradiation chambers. The
implementation of the strategies include considering each of the four aspects of the PDCA
cycle at each and every step. Also, in order to access the success rate of the implemented
strategies a comparison with respect to the database of the previous year was analysed based
on the criteria of complains from the community based near the hospital in terms of
percentage of infection, foul odour, breach of hospital policies, spreading of litter by local
animals and number of complains. The results showed a significant decrease in all the above

8RISK ASSESSMENT AND QUALITY IN HEALTH
said factors after the successful implementation of all the waste management strategies.
Therefore, it can be said that in order to access the level of efficient planning and
implementation of successful quality control policies, the first step would involve the
identification of the existing lacuna that needs to be taken immediate care of. The second step
would involve the identification of the objectives and the requisite planning required to take
up a course of action. Once the planning is done, the next step involves successful
implementation of the designed strategies and finally enforcing the strategies under strict
supervision of the quality control department. Therefore for the successful operation of
business strategy it is important to address all the necessary requisites and then develop a
course of action.
References:
Bernardo, M., Simon, A., Tarí, J. J., and Molina-Azorín, J. F. 2015. Benefits of management
systems integration: a literature review. Journal of Cleaner Production, 94, 260-267.
Clark, D. M., Silvester, K., and Knowles, S. 2013. Lean management systems: creating a
culture of continuous quality improvement. Journal of clinical pathology, jclinpath-2013.
De Oliveira, O. J. 2013. Guidelines for the integration of certifiable management systems in
industrial companies. Journal of Cleaner Production, 57, 124-133.
Johnson, C. N. 2016. The benefits of PDCA. Quality Progress, 49(1), 45.
Matsuo, M., and Nakahara, J. 2013. The effects of the PDCA cycle and OJT on workplace
learning. The International Journal of Human Resource Management, 24(1), 195-207.
Pietrzak, M., and Paliszkiewicz, J. 2015. Framework of Strategic Learning: The PDCA
Cycle. Management (18544223), 10(2).
said factors after the successful implementation of all the waste management strategies.
Therefore, it can be said that in order to access the level of efficient planning and
implementation of successful quality control policies, the first step would involve the
identification of the existing lacuna that needs to be taken immediate care of. The second step
would involve the identification of the objectives and the requisite planning required to take
up a course of action. Once the planning is done, the next step involves successful
implementation of the designed strategies and finally enforcing the strategies under strict
supervision of the quality control department. Therefore for the successful operation of
business strategy it is important to address all the necessary requisites and then develop a
course of action.
References:
Bernardo, M., Simon, A., Tarí, J. J., and Molina-Azorín, J. F. 2015. Benefits of management
systems integration: a literature review. Journal of Cleaner Production, 94, 260-267.
Clark, D. M., Silvester, K., and Knowles, S. 2013. Lean management systems: creating a
culture of continuous quality improvement. Journal of clinical pathology, jclinpath-2013.
De Oliveira, O. J. 2013. Guidelines for the integration of certifiable management systems in
industrial companies. Journal of Cleaner Production, 57, 124-133.
Johnson, C. N. 2016. The benefits of PDCA. Quality Progress, 49(1), 45.
Matsuo, M., and Nakahara, J. 2013. The effects of the PDCA cycle and OJT on workplace
learning. The International Journal of Human Resource Management, 24(1), 195-207.
Pietrzak, M., and Paliszkiewicz, J. 2015. Framework of Strategic Learning: The PDCA
Cycle. Management (18544223), 10(2).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9RISK ASSESSMENT AND QUALITY IN HEALTH
Runciman, B., Merry, A., and Walton, M. 2017. Safety and ethics in healthcare: a guide to
getting it right. CRC Press.
Salman, R. A. S., Beller, E., Kagan, J., Hemminki, E., Phillips, R. S., Savulescu, J., ... and
Chalmers, I. 2014. Increasing value and reducing waste in biomedical research regulation and
management. The Lancet, 383(9912), 176-185.
Woldgebriel, S., Kitaw, D., and Beshah, B. 2014. Quality improvement approaches and
models in healthcare. Industrial Engineering & Management, 3(03), 2169-0316.
Runciman, B., Merry, A., and Walton, M. 2017. Safety and ethics in healthcare: a guide to
getting it right. CRC Press.
Salman, R. A. S., Beller, E., Kagan, J., Hemminki, E., Phillips, R. S., Savulescu, J., ... and
Chalmers, I. 2014. Increasing value and reducing waste in biomedical research regulation and
management. The Lancet, 383(9912), 176-185.
Woldgebriel, S., Kitaw, D., and Beshah, B. 2014. Quality improvement approaches and
models in healthcare. Industrial Engineering & Management, 3(03), 2169-0316.
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




