Risk Management in Clinical Studies: Identifying and Mitigating Risks
VerifiedAdded on 2022/08/23
|11
|2075
|30
Report
AI Summary
This report provides a comprehensive overview of risk management in clinical studies. It begins with an introduction to risk management, defining its importance and purpose within the context of clinical research. The report identifies various risks inherent in clinical studies, including those related to sponsors, specialist availability, regulatory compliance, infrastructure, data collection, and costs. A conceptual framework is presented to illustrate the relationships between risk management and clinical study components. The methodology section outlines the use of primary and secondary data sources, with a focus on quantitative data analysis. The significance of effective risk management in clinical studies is emphasized, highlighting the need for proactive identification, evaluation, and mitigation strategies. The report references relevant literature on risk management and its application in various fields, providing a foundation for understanding the challenges and best practices in clinical research. This report aims to equip researchers with the knowledge necessary to navigate the complexities of clinical studies and ensure their successful completion.

Running head: RISK MANAGEMENT
RISK MANAGEMENT
Name of the Student:
Name of the University:
Author note:
RISK MANAGEMENT
Name of the Student:
Name of the University:
Author note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1RISK MANAGEMENT
Table of Contents
Risk Management in Clinical Study:...............................................................................................2
Introduction:....................................................................................................................................2
Purpose of the Research:.............................................................................................................2
Literature review:.............................................................................................................................2
Concept of Risk Management:....................................................................................................2
Risks in the Clinical Practise:......................................................................................................4
Conceptual Frame Work:.................................................................................................................6
Sample:............................................................................................................................................7
Methodology....................................................................................................................................7
Analysis:..........................................................................................................................................7
Significance:....................................................................................................................................8
Bibliography....................................................................................................................................9
Table of Contents
Risk Management in Clinical Study:...............................................................................................2
Introduction:....................................................................................................................................2
Purpose of the Research:.............................................................................................................2
Literature review:.............................................................................................................................2
Concept of Risk Management:....................................................................................................2
Risks in the Clinical Practise:......................................................................................................4
Conceptual Frame Work:.................................................................................................................6
Sample:............................................................................................................................................7
Methodology....................................................................................................................................7
Analysis:..........................................................................................................................................7
Significance:....................................................................................................................................8
Bibliography....................................................................................................................................9

2RISK MANAGEMENT
Risk Management in Clinical Study:
Introduction:
Any business and any project have distortions, challenges, and changes due to the
influence of external factors, which are continually changing or internal ones, which are due to
the different changes that occur during the activity. These influences lead to the emergence of
risks and uncertainty. When carrying out projects, account must be taken of the risks that may
arise throughout them. These risks should not be ignored or hidden but they should be treated
responsibly by the project manager and his entire team. A project manager is not able to solve
every risk that appears in a project, so every person in his team has to do something about risks.
Purpose of the Research:
The purpose of the research is to identify the importance of Risk Management and its
implementation in the field of clinical studies.
In order to perform the clinical study, there are numerous issues which can interrupt the
process if the clinical study. In order to prevent these interruptions, risk analysis is important. In
this research we will identify the risks in clinical studies and add certain ways in which they can
be mitigated.
Literature review:
Concept of Risk Management:
Project risk is an uncertain event or condition that, if it occurs, affects at least one project
objective. The risk deals with the uncertainty of the events that could affect the project. Some
potential negative events of the project are highly likely to occur on specific projects. Examples
are:
Risk Management in Clinical Study:
Introduction:
Any business and any project have distortions, challenges, and changes due to the
influence of external factors, which are continually changing or internal ones, which are due to
the different changes that occur during the activity. These influences lead to the emergence of
risks and uncertainty. When carrying out projects, account must be taken of the risks that may
arise throughout them. These risks should not be ignored or hidden but they should be treated
responsibly by the project manager and his entire team. A project manager is not able to solve
every risk that appears in a project, so every person in his team has to do something about risks.
Purpose of the Research:
The purpose of the research is to identify the importance of Risk Management and its
implementation in the field of clinical studies.
In order to perform the clinical study, there are numerous issues which can interrupt the
process if the clinical study. In order to prevent these interruptions, risk analysis is important. In
this research we will identify the risks in clinical studies and add certain ways in which they can
be mitigated.
Literature review:
Concept of Risk Management:
Project risk is an uncertain event or condition that, if it occurs, affects at least one project
objective. The risk deals with the uncertainty of the events that could affect the project. Some
potential negative events of the project are highly likely to occur on specific projects. Examples
are:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3RISK MANAGEMENT
Safety risks are common for construction projects;
Changes in the value of local currency during a project affect the purchasing
power and budgets of projects with large international components;
Weather-dependent projects, such as road construction or coastal projects, risk
delays due to exceptionally wet or windy weather.
The risks of the project are separated from the organizational risks that are associated
with the business purpose of the project. The main differences of the project risk with the
dangers of the organization are:
The risk of the project is the possibility that the project events will not take place
as planned or that unplanned events will occur that will have a negative impact on
the project.
Known risks can be identified prior to their occurrence, while unknown risks are
unforeseen.
Organizational risks are associated with the business purpose of the project and
assumed by the client when deciding to do the project.
Projects by their nature are risky, so the project manager has a key role in identifying,
planning, and managing risks. There are many types of project risks. These risks can lead to cost,
scheduling, or performance issues and may create other types of adverse consequences for the
organization. Cost risk, Program Risk, Performance Risk, Governance Risk, Strategic risk,
Operational Risk, Market Risk, Legal Risk and many more.
Risk management focuses on identifying and evaluating risks for the project and
managing their chances to minimize the impact on the project. There are no projects without
Safety risks are common for construction projects;
Changes in the value of local currency during a project affect the purchasing
power and budgets of projects with large international components;
Weather-dependent projects, such as road construction or coastal projects, risk
delays due to exceptionally wet or windy weather.
The risks of the project are separated from the organizational risks that are associated
with the business purpose of the project. The main differences of the project risk with the
dangers of the organization are:
The risk of the project is the possibility that the project events will not take place
as planned or that unplanned events will occur that will have a negative impact on
the project.
Known risks can be identified prior to their occurrence, while unknown risks are
unforeseen.
Organizational risks are associated with the business purpose of the project and
assumed by the client when deciding to do the project.
Projects by their nature are risky, so the project manager has a key role in identifying,
planning, and managing risks. There are many types of project risks. These risks can lead to cost,
scheduling, or performance issues and may create other types of adverse consequences for the
organization. Cost risk, Program Risk, Performance Risk, Governance Risk, Strategic risk,
Operational Risk, Market Risk, Legal Risk and many more.
Risk management focuses on identifying and evaluating risks for the project and
managing their chances to minimize the impact on the project. There are no projects without
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4RISK MANAGEMENT
risks because there is an infinite number of events that can have a negative effect on the project.
Risk management does not refer to the elimination of risk, but the identification, evaluation, and
management of risk.
Risks in the Clinical Practise:
As discussed earlier, risks is inevitable in any project. Thus for conducting a research in
the clinical study, the following risks can occur.
Sponsor: For conducting a research in the field of the clinical study, sponsorship is
important. A study cannot be conducted without the help of finance. There could be a lack of
fund providers for the research which may again be a challenge for the researcher for completion
of the project.
Unavailability specialists: The clinical study is based upon a particular area of the
research. Hence, it is extremely important for the researcher to stay in contact with a specialist
who is expected to help him or her in the research. However, the availability of the specialist
depends upon the circumstances and hence unavailability of the specialist can be a huge problem
for the research.
Rules and regulations: The complexity of the rules and regulations are increasing with
every passing days. As the rate of frauds have increased in the clinical sectors. Hence, coping up
with them becomes difficult.
Infrastructure: the unavailability of the research infrastructure is one of the major
problems in the research field. In the clinical sector it is a great challenge as well. Study cannot
be conducted without proper equipment.
risks because there is an infinite number of events that can have a negative effect on the project.
Risk management does not refer to the elimination of risk, but the identification, evaluation, and
management of risk.
Risks in the Clinical Practise:
As discussed earlier, risks is inevitable in any project. Thus for conducting a research in
the clinical study, the following risks can occur.
Sponsor: For conducting a research in the field of the clinical study, sponsorship is
important. A study cannot be conducted without the help of finance. There could be a lack of
fund providers for the research which may again be a challenge for the researcher for completion
of the project.
Unavailability specialists: The clinical study is based upon a particular area of the
research. Hence, it is extremely important for the researcher to stay in contact with a specialist
who is expected to help him or her in the research. However, the availability of the specialist
depends upon the circumstances and hence unavailability of the specialist can be a huge problem
for the research.
Rules and regulations: The complexity of the rules and regulations are increasing with
every passing days. As the rate of frauds have increased in the clinical sectors. Hence, coping up
with them becomes difficult.
Infrastructure: the unavailability of the research infrastructure is one of the major
problems in the research field. In the clinical sector it is a great challenge as well. Study cannot
be conducted without proper equipment.

5RISK MANAGEMENT
Difficulties in the collection of the data: the medical records and the health records are
often misused by the people for faking identities. Thus it is not always provided by the hospitals
to the student. This causes hindrances in the project data analysis as it cannot be performed
without data.
Cost: Last and the most important of all is the expense. The expense of the clinical study
is very high, which is challenging for the students.
The lack of clarity among investigators regarding the roles and responsibilities of
different oversight bodies. In focus groups with the investigators polled, it became clear that IRB
missions can be difficult to interpret. Institutions have used IRBs for risk management above and
beyond what is required for human subjects’ research, and included in their purview travel
policies, conflicts of interest, and other management issues.
Difficulties in the collection of the data: the medical records and the health records are
often misused by the people for faking identities. Thus it is not always provided by the hospitals
to the student. This causes hindrances in the project data analysis as it cannot be performed
without data.
Cost: Last and the most important of all is the expense. The expense of the clinical study
is very high, which is challenging for the students.
The lack of clarity among investigators regarding the roles and responsibilities of
different oversight bodies. In focus groups with the investigators polled, it became clear that IRB
missions can be difficult to interpret. Institutions have used IRBs for risk management above and
beyond what is required for human subjects’ research, and included in their purview travel
policies, conflicts of interest, and other management issues.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
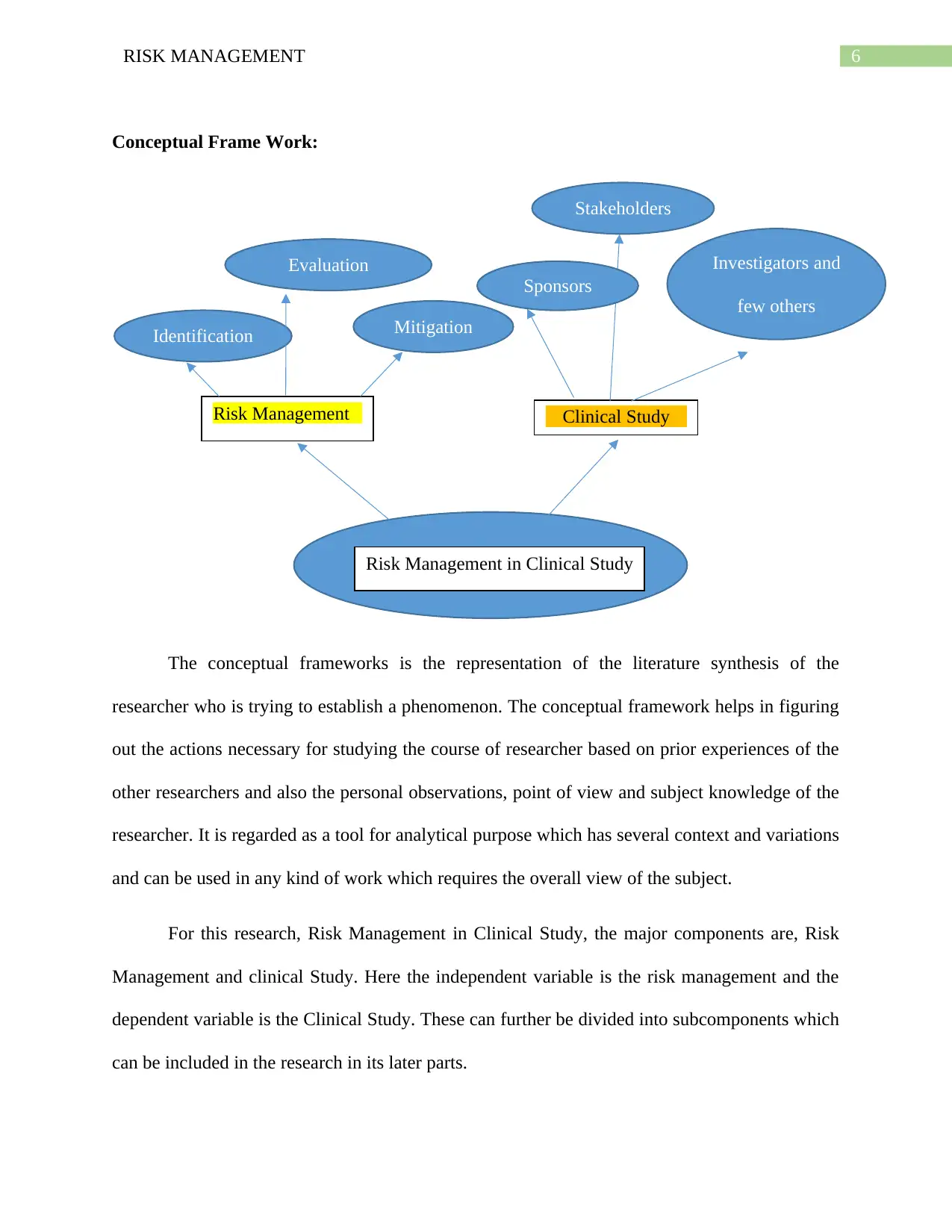
6RISK MANAGEMENT
Conceptual Frame Work:
The conceptual frameworks is the representation of the literature synthesis of the
researcher who is trying to establish a phenomenon. The conceptual framework helps in figuring
out the actions necessary for studying the course of researcher based on prior experiences of the
other researchers and also the personal observations, point of view and subject knowledge of the
researcher. It is regarded as a tool for analytical purpose which has several context and variations
and can be used in any kind of work which requires the overall view of the subject.
For this research, Risk Management in Clinical Study, the major components are, Risk
Management and clinical Study. Here the independent variable is the risk management and the
dependent variable is the Clinical Study. These can further be divided into subcomponents which
can be included in the research in its later parts.
Risk Management in Clinical Study
Risk Management Clinical Study
Identification
Evaluation
Mitigation
Sponsors
Stakeholders
Investigators and
few others
Conceptual Frame Work:
The conceptual frameworks is the representation of the literature synthesis of the
researcher who is trying to establish a phenomenon. The conceptual framework helps in figuring
out the actions necessary for studying the course of researcher based on prior experiences of the
other researchers and also the personal observations, point of view and subject knowledge of the
researcher. It is regarded as a tool for analytical purpose which has several context and variations
and can be used in any kind of work which requires the overall view of the subject.
For this research, Risk Management in Clinical Study, the major components are, Risk
Management and clinical Study. Here the independent variable is the risk management and the
dependent variable is the Clinical Study. These can further be divided into subcomponents which
can be included in the research in its later parts.
Risk Management in Clinical Study
Risk Management Clinical Study
Identification
Evaluation
Mitigation
Sponsors
Stakeholders
Investigators and
few others
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7RISK MANAGEMENT
Sample:
The subjects of the research are the researchers who conducted the study. The principal
investigator, investigators, research co-ordinator and research nurse.
The subjects are recruited on the basis of their interest towards the research, with the help of the
notice.
Methodology:
This is one of the most important part of the research which gives the researcher insights
regarding how the research has been undertaken.
There are two sources from which the data can be gathered for the purpose of the
research. They are, primary sources of data and the secondary source of data. The primary source
of data are the ones which are gathered with the help of the interviews and surveys. These are
most reliable sources of data. The secondary sources of the data are the collected from the
journal articles and books or internet sources. However, for this research we have considered the
primary sources of the data.
Analysis:
There are two ways in which data can be analyzed in a research. They are the qualitative
data analysis and the quantitative data analysis. The qualitative data analysis technique is a
broader way of analysing data from the thoughts of the people based on the secondary sources of
data mainly. The quantitative data analysis technique is a numeric approach of analyzing a data.
For this analysis technique, a data set is required for performing analysis; where the data set
consists of the responses generated after performing a survey or an interview. For this research
Sample:
The subjects of the research are the researchers who conducted the study. The principal
investigator, investigators, research co-ordinator and research nurse.
The subjects are recruited on the basis of their interest towards the research, with the help of the
notice.
Methodology:
This is one of the most important part of the research which gives the researcher insights
regarding how the research has been undertaken.
There are two sources from which the data can be gathered for the purpose of the
research. They are, primary sources of data and the secondary source of data. The primary source
of data are the ones which are gathered with the help of the interviews and surveys. These are
most reliable sources of data. The secondary sources of the data are the collected from the
journal articles and books or internet sources. However, for this research we have considered the
primary sources of the data.
Analysis:
There are two ways in which data can be analyzed in a research. They are the qualitative
data analysis and the quantitative data analysis. The qualitative data analysis technique is a
broader way of analysing data from the thoughts of the people based on the secondary sources of
data mainly. The quantitative data analysis technique is a numeric approach of analyzing a data.
For this analysis technique, a data set is required for performing analysis; where the data set
consists of the responses generated after performing a survey or an interview. For this research

8RISK MANAGEMENT
we will consider quantitative analysis of data. Although it is a complicated process, the accuracy
of the analysis is high.
Significance:
One of the essential activities in project management is project risk management.
Different risks may arise at each stage of a project. They must be identified, defined, registered,
and reported to the stakeholders. Also, these risks must be managed responsibly so that the
projects are not in danger of being closed; to record costs higher than those budgeted; not to
complete the deliverables or not to have delivered the project on time. However, the project
manager can use several tools, such as „the Exposure-probability matrix of risk in projects” and
„the pattern to record the anticipated risks of the project” for ease of risk management. It may
also use guidelines to analyze risks: the likelihood of risk occurrence, the impact of the risk, the
exposure to risk or risk score, and the period of appearance of the risk, to take appropriate
measures can be made to mitigate them.
we will consider quantitative analysis of data. Although it is a complicated process, the accuracy
of the analysis is high.
Significance:
One of the essential activities in project management is project risk management.
Different risks may arise at each stage of a project. They must be identified, defined, registered,
and reported to the stakeholders. Also, these risks must be managed responsibly so that the
projects are not in danger of being closed; to record costs higher than those budgeted; not to
complete the deliverables or not to have delivered the project on time. However, the project
manager can use several tools, such as „the Exposure-probability matrix of risk in projects” and
„the pattern to record the anticipated risks of the project” for ease of risk management. It may
also use guidelines to analyze risks: the likelihood of risk occurrence, the impact of the risk, the
exposure to risk or risk score, and the period of appearance of the risk, to take appropriate
measures can be made to mitigate them.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9RISK MANAGEMENT
Bibliography:
Choi, T. M., Chiu, C. H., & Chan, H. K. (2016). Risk management of logistics systems.
Fan, Y., & Stevenson, M. (2018). A review of supply chain risk management: definition, theory,
and research agenda. International Journal of Physical Distribution & Logistics
Management.
Glendon, A. I., Clarke, S., & McKenna, E. (2016). Human safety and risk management. Crc
Press.
Hopkin, P. (2018). Fundamentals of risk management: understanding, evaluating and
implementing effective risk management. Kogan Page Publishers.
Hubbard, D. W. (2020). The failure of risk management: Why it's broken and how to fix it. John
Wiley & Sons.
Hubbard, D. W. (2020). The failure of risk management: Why it's broken and how to fix it. John
Wiley & Sons.
Jørgensen, L., Doshi, P., Gøtzsche, P., & Jefferson, T. (2018). Challenges of independent
assessment of potential harms of HPV vaccines. Bmj, 362, k3694.
Liang, J., Zhong, M., Zeng, G., Chen, G., Hua, S., Li, X., ... & Gao, X. (2017). Risk management
for optimal land use planning integrating ecosystem services values: A case study in
Changsha, Middle China. Science of the Total Environment, 579, 1675-1682.
Sadgrove, K. (2016). The complete guide to business risk management. Routledge.
Zhang, X. L., Chen, M., Zhu, L. L., & Zhou, Q. (2017). Therapeutic risk and benefits of
concomitantly using herbal medicines and conventional medicines: from the perspectives
Bibliography:
Choi, T. M., Chiu, C. H., & Chan, H. K. (2016). Risk management of logistics systems.
Fan, Y., & Stevenson, M. (2018). A review of supply chain risk management: definition, theory,
and research agenda. International Journal of Physical Distribution & Logistics
Management.
Glendon, A. I., Clarke, S., & McKenna, E. (2016). Human safety and risk management. Crc
Press.
Hopkin, P. (2018). Fundamentals of risk management: understanding, evaluating and
implementing effective risk management. Kogan Page Publishers.
Hubbard, D. W. (2020). The failure of risk management: Why it's broken and how to fix it. John
Wiley & Sons.
Hubbard, D. W. (2020). The failure of risk management: Why it's broken and how to fix it. John
Wiley & Sons.
Jørgensen, L., Doshi, P., Gøtzsche, P., & Jefferson, T. (2018). Challenges of independent
assessment of potential harms of HPV vaccines. Bmj, 362, k3694.
Liang, J., Zhong, M., Zeng, G., Chen, G., Hua, S., Li, X., ... & Gao, X. (2017). Risk management
for optimal land use planning integrating ecosystem services values: A case study in
Changsha, Middle China. Science of the Total Environment, 579, 1675-1682.
Sadgrove, K. (2016). The complete guide to business risk management. Routledge.
Zhang, X. L., Chen, M., Zhu, L. L., & Zhou, Q. (2017). Therapeutic risk and benefits of
concomitantly using herbal medicines and conventional medicines: from the perspectives
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10RISK MANAGEMENT
of evidence based on randomized controlled trials and clinical risk
management. Evidence-Based Complementary and Alternative Medicine, 2017.
of evidence based on randomized controlled trials and clinical risk
management. Evidence-Based Complementary and Alternative Medicine, 2017.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





