Case Study: Managing Risk in a Pharmaceutical Company Setting
VerifiedAdded on 2022/12/27
|11
|2676
|27
Case Study
AI Summary
This case study examines risk management within Dechra Pharmaceuticals, a pharmaceutical company dealing with research, development, and the sale of medicines. The report focuses on assessing the risks associated with hazardous chemicals, particularly ethidium bromide and levothyroxine, and their impact on worker safety. It involves identifying hazards, determining those at risk, developing a risk register and matrix, and analyzing the robustness of the risk assessment plan. The study highlights the importance of worker awareness, leadership commitment, and technological solutions in mitigating risks. The risk register includes details on the likelihood and impact of hazards, severity, and mitigation actions, such as the use of specialized equipment and safety protocols. The efficacy of the risk assessment plan is evaluated, emphasizing improvements in safety measures and worker awareness. The conclusion underscores the importance of continuous improvement in risk management to ensure worker safety and health in a pharmaceutical environment.

Managing Risk
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
INTRODUCTION...........................................................................................................................3
Identification of the hazards present and those at risk from hazards ........................................3
Developing risk register and risk matrix.....................................................................................5
Analysis of the robustness of the risk assessment plan to reduce the errors and mitigate the
risk..............................................................................................................................................8
Analysis of technology role in using information to reduce the errors.......................................9
Evaluate the efficacy of the risk assessment plan.......................................................................9
CONCLUSION ............................................................................................................................10
REFERENCES..............................................................................................................................11
INTRODUCTION...........................................................................................................................3
Identification of the hazards present and those at risk from hazards ........................................3
Developing risk register and risk matrix.....................................................................................5
Analysis of the robustness of the risk assessment plan to reduce the errors and mitigate the
risk..............................................................................................................................................8
Analysis of technology role in using information to reduce the errors.......................................9
Evaluate the efficacy of the risk assessment plan.......................................................................9
CONCLUSION ............................................................................................................................10
REFERENCES..............................................................................................................................11

INTRODUCTION
Risk assessment is the method or framework which involve various task and process for
the identification of hazards and risk factors, which can harm the peoples at work place and
organisation. This method is divided into five steps which start from the analysis of risk and
hazard, identification of peoples who are at risk and development of the risk management
strategies, then its final step is evaluation of the risk management plan effects. This process is
continuously done in order to asses the risk of every new techniques, substance and harm related
to that and to understand positive impact of risk management strategies so that it can be improve
or update if not work effective. Royal Croatian–Slavonian Bacteriological Institute is now the
part of Dechra Pharmaceuticals PLC Group. This organisation is dealing with research and
development along with marketing and sealing of the medicines. This report is involving the risk
assessment of hazardous chemicals in Dechra Pharmaceuticals (Hillson and Simon,2020).
Identification of the hazards present and those at risk from hazards
Risk assessment is one of the most important tool for assurance of workers safety and
health in work place. In this project risk assessment is use for the pharmaceutical organisation
risk analyses and management. So there are the major risk related to the chemical and tools of
the product development. Risk assessment start from the analysis of hazards, this is the first step
of the risk assessment. Then second step is identification of person who is on the risk by
selective hazard. Although there are some other more important tools which use under this
process of risk analysis which will discuss in the next step. So there is the analysis of risk related
to the the selected organisation and identification of its impact on workers. In context to the case
study organisation have already did risk assessment in their workplace for preventing the
chemical practices related hazard, but it was not successful due to the inappropriate information
and knowledge of the workers related to the chemicals. There was the absence of toxicological
and health and safety information (Durst, 2018). Due to which data sheets for safety and health
measures, was not sufficient and quality through which workers can take optional decision.
Organisation major focus is on the risk of chemical hazard on their workers, and so that's way
they want to develop the risk management plan for the hazards of chemicals. One of the most
hazardous chemical in Pharmaceuticals is company is ethidium bromide, this is the most
common chemical use in the pharmaceutical and research company. This is fluorescent tag use
as the molecule marker in the process of electrophoresis for visualisation of DNA bands.
Risk assessment is the method or framework which involve various task and process for
the identification of hazards and risk factors, which can harm the peoples at work place and
organisation. This method is divided into five steps which start from the analysis of risk and
hazard, identification of peoples who are at risk and development of the risk management
strategies, then its final step is evaluation of the risk management plan effects. This process is
continuously done in order to asses the risk of every new techniques, substance and harm related
to that and to understand positive impact of risk management strategies so that it can be improve
or update if not work effective. Royal Croatian–Slavonian Bacteriological Institute is now the
part of Dechra Pharmaceuticals PLC Group. This organisation is dealing with research and
development along with marketing and sealing of the medicines. This report is involving the risk
assessment of hazardous chemicals in Dechra Pharmaceuticals (Hillson and Simon,2020).
Identification of the hazards present and those at risk from hazards
Risk assessment is one of the most important tool for assurance of workers safety and
health in work place. In this project risk assessment is use for the pharmaceutical organisation
risk analyses and management. So there are the major risk related to the chemical and tools of
the product development. Risk assessment start from the analysis of hazards, this is the first step
of the risk assessment. Then second step is identification of person who is on the risk by
selective hazard. Although there are some other more important tools which use under this
process of risk analysis which will discuss in the next step. So there is the analysis of risk related
to the the selected organisation and identification of its impact on workers. In context to the case
study organisation have already did risk assessment in their workplace for preventing the
chemical practices related hazard, but it was not successful due to the inappropriate information
and knowledge of the workers related to the chemicals. There was the absence of toxicological
and health and safety information (Durst, 2018). Due to which data sheets for safety and health
measures, was not sufficient and quality through which workers can take optional decision.
Organisation major focus is on the risk of chemical hazard on their workers, and so that's way
they want to develop the risk management plan for the hazards of chemicals. One of the most
hazardous chemical in Pharmaceuticals is company is ethidium bromide, this is the most
common chemical use in the pharmaceutical and research company. This is fluorescent tag use
as the molecule marker in the process of electrophoresis for visualisation of DNA bands.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Another hazardous chemical is levothyroxine but it has very minimum harm in comparison to
ethidium bromide . Due to the higher exposer to levothyroxine can cause hyperthyroidism.
Workers and researchers who work in the production of pharmaceutical product and research is
at the high risk of these hazardous chemicals (Treasure, 2017).
Developing risk register and risk matrix
Risk register
Risk register is the information and risk management tool it helps to analyse and mange
the potential risk elements with the aid of proper safety measures . Nature of the risk, references
and owners is analyse and manage by the tool. So risk register is explain below in context to
organisation and its development for risk management. This risk register is the risk management
tool which help to conduct the risk management effectively. Its major role is that it provide the
formate to conduct the risk assessment and manage the information, of risk and action for
mitigation along with the information of responsible person for its management (Finger, 2018).
ID Risk Likelihoo Impact of severity owner Mitigating
ethidium bromide . Due to the higher exposer to levothyroxine can cause hyperthyroidism.
Workers and researchers who work in the production of pharmaceutical product and research is
at the high risk of these hazardous chemicals (Treasure, 2017).
Developing risk register and risk matrix
Risk register
Risk register is the information and risk management tool it helps to analyse and mange
the potential risk elements with the aid of proper safety measures . Nature of the risk, references
and owners is analyse and manage by the tool. So risk register is explain below in context to
organisation and its development for risk management. This risk register is the risk management
tool which help to conduct the risk management effectively. Its major role is that it provide the
formate to conduct the risk assessment and manage the information, of risk and action for
mitigation along with the information of responsible person for its management (Finger, 2018).
ID Risk Likelihoo Impact of severity owner Mitigating
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

description d of the
risk
the risk action
1 Ethidium
bromide, is
mutagenic and
carcinogenic
Possibility
is low or
medium.
High
It can
cause
mutagenes
is and
cancer in
the
workers
who use it
for the
work.
High This risk
is should
be manage
by the
manager
of the
organisati
on and by
users to
where
they have
to take
precaution
s. inviting
specialist
experts
along with
the
worekrs.
GelRed
nucleic acid
gel stain.
This is use
as the
mitigation
action
where it
substitute
with the
pace of
Ethidium
bromide.
New
techniques
with
effective
masers
(Chapman,
2019).
2 levothyroxine
higher exposer
to this chemical
can cause
hyperthyroidism
(Tranter, 2020)..
Possibility
is lass.
medium
It can
cause
foetal
toxicity,
hyperthyro
idism
Medium Manger
and users.
Manger
have to
focus
about the
lass
exposer to
glove box.
heating,
ventilation
and air
conditionin
g
equipment,
such as
risk
the risk action
1 Ethidium
bromide, is
mutagenic and
carcinogenic
Possibility
is low or
medium.
High
It can
cause
mutagenes
is and
cancer in
the
workers
who use it
for the
work.
High This risk
is should
be manage
by the
manager
of the
organisati
on and by
users to
where
they have
to take
precaution
s. inviting
specialist
experts
along with
the
worekrs.
GelRed
nucleic acid
gel stain.
This is use
as the
mitigation
action
where it
substitute
with the
pace of
Ethidium
bromide.
New
techniques
with
effective
masers
(Chapman,
2019).
2 levothyroxine
higher exposer
to this chemical
can cause
hyperthyroidism
(Tranter, 2020)..
Possibility
is lass.
medium
It can
cause
foetal
toxicity,
hyperthyro
idism
Medium Manger
and users.
Manger
have to
focus
about the
lass
exposer to
glove box.
heating,
ventilation
and air
conditionin
g
equipment,
such as

that
chemical.
User have
to mange
this risk
by taking
the proper
instruction
of the
safety
(Sakhel,
2017).
filters
(HEPA)
which have
the air
filtration
inlet and
out late
through
which
environme
nt get
protected.
Risk description- This is the section manage with in the risk register, this involves the
description of the risk related thing. In this case health related harmfully effects of the chemical
is a risk for the organisation workers that is the mutation and chances of the cancer
development.
Likelihood of the risk- In this section possibility of hazard is discuss and manage such
as the probability of risk to happen. Such as in this case there is the huge chances of the cancer
and mutation from the Ethidium bromide and there is the possibility of levothyroxine harmful
effects but in some cases due to the higher exposer.
Impact of the risk- If risk can happen then what will be the impact of this on workers
is, describe in this section of the report. In the case of pharmaceutical company impact of the
chemical compound is high, which can highly effects the health of the workers.
Severity- In this section of the risk report seriousness of the risk is describe by analysing
this from the likelihood and impact of the risk. Such as in this case Ethidium bromide risk is
more sever then the levothyroxine, because it can change the life of individual with life
threatening disease (Villegas-Gonzále, 2017).
Owner- This section is use to mention the role of responsible personas for risk
management. Who has mange different measures for risk prevention. In this case of the risk
chemical.
User have
to mange
this risk
by taking
the proper
instruction
of the
safety
(Sakhel,
2017).
filters
(HEPA)
which have
the air
filtration
inlet and
out late
through
which
environme
nt get
protected.
Risk description- This is the section manage with in the risk register, this involves the
description of the risk related thing. In this case health related harmfully effects of the chemical
is a risk for the organisation workers that is the mutation and chances of the cancer
development.
Likelihood of the risk- In this section possibility of hazard is discuss and manage such
as the probability of risk to happen. Such as in this case there is the huge chances of the cancer
and mutation from the Ethidium bromide and there is the possibility of levothyroxine harmful
effects but in some cases due to the higher exposer.
Impact of the risk- If risk can happen then what will be the impact of this on workers
is, describe in this section of the report. In the case of pharmaceutical company impact of the
chemical compound is high, which can highly effects the health of the workers.
Severity- In this section of the risk report seriousness of the risk is describe by analysing
this from the likelihood and impact of the risk. Such as in this case Ethidium bromide risk is
more sever then the levothyroxine, because it can change the life of individual with life
threatening disease (Villegas-Gonzále, 2017).
Owner- This section is use to mention the role of responsible personas for risk
management. Who has mange different measures for risk prevention. In this case of the risk
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
assessment inviting specialist experts along with the workers for hierarchy of prevention
measures and high-level management commitment along with the aid of technological support.
Mitigating action- This section involve the different risk prevention strategies and
measures of the activities and process so that workers can follow. In this case organisation have
selected various risk management strategies and measures for the use of chemical compound at
every activity. Such as they have the laminar air flow with fume hoods, central weighing room
with highly equipped facility's. Laminar air flow with (HEPA) filter. Safe storage of the
chemicals. Air-lock (PAL) special suit with protective hood with safe breathing apparatus (Van
Staveren, 2018).
All these section in report is manage so that every one can follow this by understanding
its severity and their role.
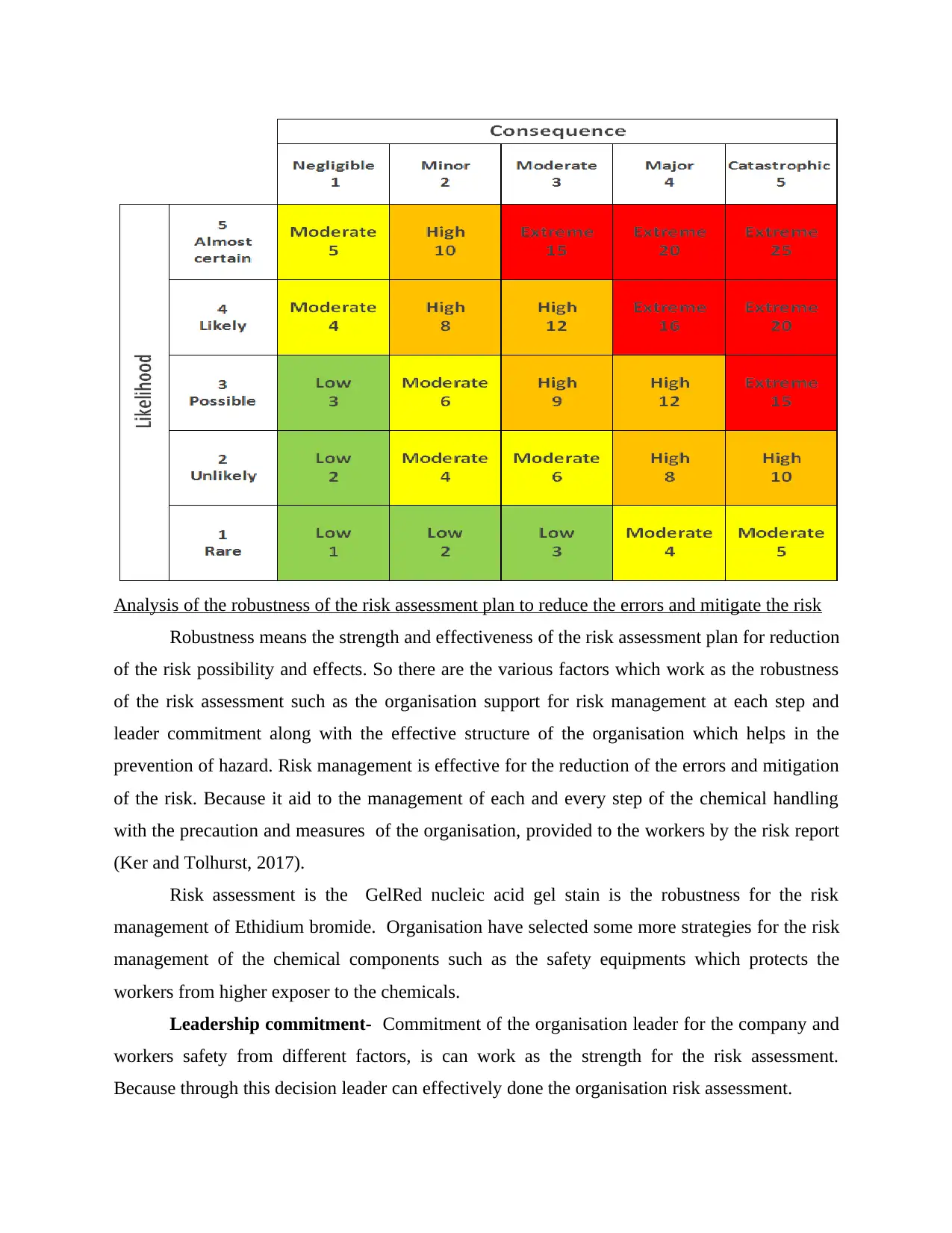
Risk matrix-
This is the matrix tool which is use during the process of risk assessment in order to
analyse the level risk possibility and level of risk severity. Risk metric is given below in context
to the selected organisation risk management.
Major Catastrophic
Possible ( Ethidium bromide) High Extreme
Likely ( levothyroxine ) Extreme Extreme
measures and high-level management commitment along with the aid of technological support.
Mitigating action- This section involve the different risk prevention strategies and
measures of the activities and process so that workers can follow. In this case organisation have
selected various risk management strategies and measures for the use of chemical compound at
every activity. Such as they have the laminar air flow with fume hoods, central weighing room
with highly equipped facility's. Laminar air flow with (HEPA) filter. Safe storage of the
chemicals. Air-lock (PAL) special suit with protective hood with safe breathing apparatus (Van
Staveren, 2018).
All these section in report is manage so that every one can follow this by understanding
its severity and their role.
Risk matrix-
This is the matrix tool which is use during the process of risk assessment in order to
analyse the level risk possibility and level of risk severity. Risk metric is given below in context
to the selected organisation risk management.
Major Catastrophic
Possible ( Ethidium bromide) High Extreme
Likely ( levothyroxine ) Extreme Extreme
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Analysis of the robustness of the risk assessment plan to reduce the errors and mitigate the risk
Robustness means the strength and effectiveness of the risk assessment plan for reduction
of the risk possibility and effects. So there are the various factors which work as the robustness
of the risk assessment such as the organisation support for risk management at each step and
leader commitment along with the effective structure of the organisation which helps in the
prevention of hazard. Risk management is effective for the reduction of the errors and mitigation
of the risk. Because it aid to the management of each and every step of the chemical handling
with the precaution and measures of the organisation, provided to the workers by the risk report
(Ker and Tolhurst, 2017).
Risk assessment is the GelRed nucleic acid gel stain is the robustness for the risk
management of Ethidium bromide. Organisation have selected some more strategies for the risk
management of the chemical components such as the safety equipments which protects the
workers from higher exposer to the chemicals.
Leadership commitment- Commitment of the organisation leader for the company and
workers safety from different factors, is can work as the strength for the risk assessment.
Because through this decision leader can effectively done the organisation risk assessment.
Robustness means the strength and effectiveness of the risk assessment plan for reduction
of the risk possibility and effects. So there are the various factors which work as the robustness
of the risk assessment such as the organisation support for risk management at each step and
leader commitment along with the effective structure of the organisation which helps in the
prevention of hazard. Risk management is effective for the reduction of the errors and mitigation
of the risk. Because it aid to the management of each and every step of the chemical handling
with the precaution and measures of the organisation, provided to the workers by the risk report
(Ker and Tolhurst, 2017).
Risk assessment is the GelRed nucleic acid gel stain is the robustness for the risk
management of Ethidium bromide. Organisation have selected some more strategies for the risk
management of the chemical components such as the safety equipments which protects the
workers from higher exposer to the chemicals.
Leadership commitment- Commitment of the organisation leader for the company and
workers safety from different factors, is can work as the strength for the risk assessment.
Because through this decision leader can effectively done the organisation risk assessment.

Organisation infrastructure- This is the important factor which should be done in the
organisation according to the requirement of the risk assessment. If equipments and
technological system of the organisation is facilitated with in the organisation then it should aid
to the risk management (Louche, 2017).
Analysis of technology role in using information to reduce the errors
At present there are the various task, functions and substances use in the organisation for
the production and management of the company functions, each and every assets of the
organisation is having some risk for the workers some with less possibility and less effects and
some with higher possibility and sever effects. So information technology is the key to get and
provide the hazard management precaution or measures to workers and employees of the
organisation. Internet technology and online information resource's play impotent role in the
using of the correct safety measure data in order to reduce the chance of hazard (Karmakar,
2019). Through information or internet technology practitioner can get and use the evidence
based method's for the chemical handling during the performance of pharmaceutical projects.
But in terms of this risk assessment project due to the absence of the relevant data in the online
information resources, related to the chemical handling. They have faced various issues in the
management of Safety data sheets (Steele, 2018).
Evaluate the efficacy of the risk assessment plan
This is the final step of the risk assessment process which is done on the timely bases for
measurement of the risk management precautions effects on it. That how it effected the risk in
terms of prevention and control. This is also done for the identification its error so that it can be
improve with further more effective safety precautions and measures. So this risk assessment
project it has been evaluated that organisation ( Dechra group) have developed strong safety and
health, measures or precaution policy. Control over the hazardous substances has improved at
the work environment (Bernard, 2018). There is the huge improvement and development in the
workers awareness about safety and health precautions at work place. There is the huge
reduction in the number if hazards while working with hazardous chemicals. Success in risk
management has achieved by the management of huge problems and barriers. Such as they have
been faced problem in the finding of relevant data about the chemicals. So the major problem
was in the quality of toxicological and health, safety information present in the internet sources.
Poor quality of the safety data sheets provided by the suppliers. Most of the workers refused to
organisation according to the requirement of the risk assessment. If equipments and
technological system of the organisation is facilitated with in the organisation then it should aid
to the risk management (Louche, 2017).
Analysis of technology role in using information to reduce the errors
At present there are the various task, functions and substances use in the organisation for
the production and management of the company functions, each and every assets of the
organisation is having some risk for the workers some with less possibility and less effects and
some with higher possibility and sever effects. So information technology is the key to get and
provide the hazard management precaution or measures to workers and employees of the
organisation. Internet technology and online information resource's play impotent role in the
using of the correct safety measure data in order to reduce the chance of hazard (Karmakar,
2019). Through information or internet technology practitioner can get and use the evidence
based method's for the chemical handling during the performance of pharmaceutical projects.
But in terms of this risk assessment project due to the absence of the relevant data in the online
information resources, related to the chemical handling. They have faced various issues in the
management of Safety data sheets (Steele, 2018).
Evaluate the efficacy of the risk assessment plan
This is the final step of the risk assessment process which is done on the timely bases for
measurement of the risk management precautions effects on it. That how it effected the risk in
terms of prevention and control. This is also done for the identification its error so that it can be
improve with further more effective safety precautions and measures. So this risk assessment
project it has been evaluated that organisation ( Dechra group) have developed strong safety and
health, measures or precaution policy. Control over the hazardous substances has improved at
the work environment (Bernard, 2018). There is the huge improvement and development in the
workers awareness about safety and health precautions at work place. There is the huge
reduction in the number if hazards while working with hazardous chemicals. Success in risk
management has achieved by the management of huge problems and barriers. Such as they have
been faced problem in the finding of relevant data about the chemicals. So the major problem
was in the quality of toxicological and health, safety information present in the internet sources.
Poor quality of the safety data sheets provided by the suppliers. Most of the workers refused to
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

take training. So organisation have achieved success in the risk assessment of the chemical
hazard management after the hard efforts and now there is the strong safety and health measures.
For more improvement in the mitigating action organisation have planed to implement new
software for the collection of relevant information of chemical (Alston, 2017).
CONCLUSION
From the above discussion it has been concluded that risk assessment is the important
functions or method which has to be implemented by every type of the organisation in order to
protect their workers and service user from the risk hazard of the organisation process and assets.
There are the five major steps of the risk assessment which is done with in the involvement of
some tools, that is risk register and risk matrix through which all the steps of risk assessment is
can be done properly. Those five steps are analysis of the risk, and then understanding that who
at the high risk, then development of the precautions and finally step is evaluation of risk
assessment effect in order to improve it if not work properly.
hazard management after the hard efforts and now there is the strong safety and health measures.
For more improvement in the mitigating action organisation have planed to implement new
software for the collection of relevant information of chemical (Alston, 2017).
CONCLUSION
From the above discussion it has been concluded that risk assessment is the important
functions or method which has to be implemented by every type of the organisation in order to
protect their workers and service user from the risk hazard of the organisation process and assets.
There are the five major steps of the risk assessment which is done with in the involvement of
some tools, that is risk register and risk matrix through which all the steps of risk assessment is
can be done properly. Those five steps are analysis of the risk, and then understanding that who
at the high risk, then development of the precautions and finally step is evaluation of risk
assessment effect in order to improve it if not work properly.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

REFERENCES
Books and Journals
Hillson and Simon,2020. Practical project risk management: The ATOM methodology. Berrett-
Koehler Publishers.
Durst, 2018. Knowledge risk management in the public sector: insights into a Swedish
municipality. Journal of Knowledge Management.
Treasure, 2017. Diagnosis and risk management in primary care: words that count, numbers that
speak. CRC Press.
Finger, 2018. Environmental risk management and financial performance in the banking
industry: A cross-country comparison. Journal of International Financial Markets,
Institutions and Money, 52, pp.240-261.
Sakhel, 2017. Corporate climate risk management: Are European companies prepared?. Journal
of Cleaner Production, 165, pp.103-118.
Villegas-Gonzále, 2017. Territorial vulnerability assessment frame in Colombia: Disaster risk
management. International journal of disaster risk reduction, 21, pp.384-395.
Van Staveren, 2018. Uncertainty and ground conditions: a risk management approach. CRC
Press.
Ker and Tolhurst, 2017. Canadian business risk management: Private firms, crown corporations,
and public Institutions. Canadian Journal of Agricultural Economics/Revue canadienne
d'agroeconomie, 65(4), pp.591-612.
Louche, 2017. Innovative CSR: From risk management to value creation. Routledge.
Steele, 2018. Understanding suicide across the lifespan: a United States perspective of suicide
risk factors, assessment & management. Journal of forensic sciences, 63(1), pp.162-
171.
Bernard, 2018. An exploratory study of safety culture, biological risk management and hand
hygiene of healthcare professionals. Journal of advanced nursing, 74(4), pp.827-837.
Alston, 2017. How Safe is Safe Enough?: Leadership, Safety and Risk Management. Routledge.
Karmakar, 2019. Intraday portfolio risk management using VaR and CVaR: A CGARCH-EVT-
Copula approach. International Journal of Forecasting, 35(2), pp.699-709.
Chapman, 2019. The rules of project risk management: Implementation guidelines for major
projects. Routledge.
Tranter, 2020. Occupational hygiene and risk management. Routledge.
Books and Journals
Hillson and Simon,2020. Practical project risk management: The ATOM methodology. Berrett-
Koehler Publishers.
Durst, 2018. Knowledge risk management in the public sector: insights into a Swedish
municipality. Journal of Knowledge Management.
Treasure, 2017. Diagnosis and risk management in primary care: words that count, numbers that
speak. CRC Press.
Finger, 2018. Environmental risk management and financial performance in the banking
industry: A cross-country comparison. Journal of International Financial Markets,
Institutions and Money, 52, pp.240-261.
Sakhel, 2017. Corporate climate risk management: Are European companies prepared?. Journal
of Cleaner Production, 165, pp.103-118.
Villegas-Gonzále, 2017. Territorial vulnerability assessment frame in Colombia: Disaster risk
management. International journal of disaster risk reduction, 21, pp.384-395.
Van Staveren, 2018. Uncertainty and ground conditions: a risk management approach. CRC
Press.
Ker and Tolhurst, 2017. Canadian business risk management: Private firms, crown corporations,
and public Institutions. Canadian Journal of Agricultural Economics/Revue canadienne
d'agroeconomie, 65(4), pp.591-612.
Louche, 2017. Innovative CSR: From risk management to value creation. Routledge.
Steele, 2018. Understanding suicide across the lifespan: a United States perspective of suicide
risk factors, assessment & management. Journal of forensic sciences, 63(1), pp.162-
171.
Bernard, 2018. An exploratory study of safety culture, biological risk management and hand
hygiene of healthcare professionals. Journal of advanced nursing, 74(4), pp.827-837.
Alston, 2017. How Safe is Safe Enough?: Leadership, Safety and Risk Management. Routledge.
Karmakar, 2019. Intraday portfolio risk management using VaR and CVaR: A CGARCH-EVT-
Copula approach. International Journal of Forecasting, 35(2), pp.699-709.
Chapman, 2019. The rules of project risk management: Implementation guidelines for major
projects. Routledge.
Tranter, 2020. Occupational hygiene and risk management. Routledge.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





