Prevalence of Staphylococcus aureus Infection Risk in RMIT Students
VerifiedAdded on 2022/07/28
|8
|2411
|27
Report
AI Summary
This report presents a cross-sectional study conducted among RMIT University students from 2017 to 2019, investigating the prevalence of Staphylococcus aureus nasal colonization. The study involved nasal swab sampling and culturing on Mannitol salt agar, with positive cases identified through gram stain, coagulase, and catalase tests. The results revealed prevalence rates of 25% in 2017, 24% in 2018, and 18% in 2019, indicating a declining trend. The report compares these findings with global prevalence data and discusses potential risk factors such as demographic characteristics, medical history, and lifestyle factors. The study concludes with recommendations for improved hygiene practices and reduced transmission risks to mitigate the spread of Staphylococcus aureus within the university community.

Staphylococcus aureus Infection Risk among University Students of RMIT University
University
Name
Date
University
Name
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
Introduction
Staphylococcus aureus micro bacteria are part of the human flora having the ability to
colonies the anterior areas of the skin and the throat. Staphylococcus aureus has been categorized
as an opportunistic disease which can colonize and impose health threats when in favourable
conditions among healthy persons (Buchan, Foster & Renshaw, 2019). Globally the common cause of
soft skin and nosocomial infections are Staphylococcus aureus. It has been characterized by high
morbidity and mortality. Currently, Staphylococcus aureus has become a serious threat and health
issue raising concerns due to the emergence of drug-resistant strains especially the methicilin
resistant Staphylococcus aureus (Costa et al., 2017).
Globally it is estimated that it affects close to about 20%-30% of the population and long
term carries have emerged being found as part of the normal skin, and nostrils flora. Staphylococcus
aureus it is associated with various forms of illness such as pimples, folliculitis, cellulitis and abscess
among other bacteria born diseases (Williamson, Coombs & Nimmo, 2014). In Australia data shows
that about 1600 cases of hospital-associated Staphylococcus aureus bacterium have demonstrated a
rate of 0.76 persons per 10,0000. Previous assessment of indices across Australia have shown that
Staphylococcus aureus bacteria is affecting close to 61%-77% of these are community-based
infections, while 13% were MRSA, (Coombs et al., 2018).
In victoria state in the period of 2017-2019, a total of an estimate of 9000 occurrences of
Staphylococcus aureus were recorded. About 65% of these were community associated leading to an
aggregate crude after if 13.3 CA- Staphylococcus aureus per 100,000. Further methicilin associated
resistance Staphylococcus aureus was observed to affect about 4% of the cases. Incidences have
been observed to prevalent among older male patients compared to women (95% CI, 48.5–53.4)
(Imam et al., 2019).
The occurrence of methicilin resistant Staphylococcus aureus (MRSA) has been viewed as
the leading cause of infection. Health care-associated infections have been viewed to circulate wider
across health care settings with a shift now tuning to community settings. The overall rates of MRSA
infections have been shown to increase rapidly in heath care and community settings (Coombs et al.,
2014).
Community-associated infections are described as cases which are either identified in the
primary care settings or in cases where symptoms are are present in pathological samples during the
first 48 hours of hospitalization. Comparing both health care and community-based infection
demonstrating that there is an increased risk of metastatic seeding which decreases the levels of anti
Introduction
Staphylococcus aureus micro bacteria are part of the human flora having the ability to
colonies the anterior areas of the skin and the throat. Staphylococcus aureus has been categorized
as an opportunistic disease which can colonize and impose health threats when in favourable
conditions among healthy persons (Buchan, Foster & Renshaw, 2019). Globally the common cause of
soft skin and nosocomial infections are Staphylococcus aureus. It has been characterized by high
morbidity and mortality. Currently, Staphylococcus aureus has become a serious threat and health
issue raising concerns due to the emergence of drug-resistant strains especially the methicilin
resistant Staphylococcus aureus (Costa et al., 2017).
Globally it is estimated that it affects close to about 20%-30% of the population and long
term carries have emerged being found as part of the normal skin, and nostrils flora. Staphylococcus
aureus it is associated with various forms of illness such as pimples, folliculitis, cellulitis and abscess
among other bacteria born diseases (Williamson, Coombs & Nimmo, 2014). In Australia data shows
that about 1600 cases of hospital-associated Staphylococcus aureus bacterium have demonstrated a
rate of 0.76 persons per 10,0000. Previous assessment of indices across Australia have shown that
Staphylococcus aureus bacteria is affecting close to 61%-77% of these are community-based
infections, while 13% were MRSA, (Coombs et al., 2018).
In victoria state in the period of 2017-2019, a total of an estimate of 9000 occurrences of
Staphylococcus aureus were recorded. About 65% of these were community associated leading to an
aggregate crude after if 13.3 CA- Staphylococcus aureus per 100,000. Further methicilin associated
resistance Staphylococcus aureus was observed to affect about 4% of the cases. Incidences have
been observed to prevalent among older male patients compared to women (95% CI, 48.5–53.4)
(Imam et al., 2019).
The occurrence of methicilin resistant Staphylococcus aureus (MRSA) has been viewed as
the leading cause of infection. Health care-associated infections have been viewed to circulate wider
across health care settings with a shift now tuning to community settings. The overall rates of MRSA
infections have been shown to increase rapidly in heath care and community settings (Coombs et al.,
2014).
Community-associated infections are described as cases which are either identified in the
primary care settings or in cases where symptoms are are present in pathological samples during the
first 48 hours of hospitalization. Comparing both health care and community-based infection
demonstrating that there is an increased risk of metastatic seeding which decreases the levels of anti

3
microbes to act and be effective observed in community-based infections. Among these patients,
they are likely to require intensive care attention to enhance the recovery process (Tong et al.,
2015).
Associated virulent factors entail enzymes, toxins and biofilm. S. aureus has the ability to
produce various enzymes such as coagulase which can clot on the plasma of the bacterial cells thus
preventing the action of phagocytosis. The hyaluronidase has the spreading factor enabling it to
produce deoxyribonuclease, facilitating the breakdown of the DNA. The lipase digests the lipids
while the staphylokinase dissolves the fibrin aiding the spread allowing for the ability to rest drugs ().
In toxin mechanism, S. aureus has the ability to secrete exotoxins which are linked to various specific
diseases. Superantigens causes nausea, vomiting, diarrhea and abdominal pain. The exfoliative
toxins have the ability to cause staphylococcal scalded skin syndrome. Biofilms are an essential to
group of microorganism which has the ability to grow in wet surfaces. S. aureus biofilm posses the
ability to be resistant to antibiotic management and has the ability to hosts immune response.
Incurring effect entails less effectiveness for S. aureus due to the underlying distribution of antigen
(Archer et al., 2011).
The underlying carriage factors have been a fundamental avenue especially in hospital-
acquired infections and community-acquired infections. Studies have demonstrated that S. aureus is
often present in the skin of the host while a large portion is found in the nasal passage (). The
observed ability for harbouring of the S. aureus has been shown to be demonstrated by weakened
immune systems and ability to evade the innate immunity of the hosts ().
The relevance of S. aureus is an essential aspect. The increased risks in health care settings
have been linked to increased health care cost and longer hospital stay (Nelson et al., 2018). In the
health care settings, it has been a key issue of concern whether in hospitals or medical laboratories.
S. aureus is often transmitted in hospital care settings leading to an immense danger on both the
health care staff and the engaged in testing and diagnosis in laboratory settings. For this purpose the
need to conduct a population-based study among college studies on the positive occurrence of S.
aureus underlying risks factors is essential.
Materials and Methods
This cross-sectional study was conducted among RMIT university students who have been
on the campus for a period of three years in different semesters from 2017 to 2019 enrolled in both
semesters. A total of 909 studies were tested in the study. Nasal sampling was undertaken through
sterile swabs inserted into nostrils for about 1.5 cm in depth and rotated in the nostrils. The swabs
microbes to act and be effective observed in community-based infections. Among these patients,
they are likely to require intensive care attention to enhance the recovery process (Tong et al.,
2015).
Associated virulent factors entail enzymes, toxins and biofilm. S. aureus has the ability to
produce various enzymes such as coagulase which can clot on the plasma of the bacterial cells thus
preventing the action of phagocytosis. The hyaluronidase has the spreading factor enabling it to
produce deoxyribonuclease, facilitating the breakdown of the DNA. The lipase digests the lipids
while the staphylokinase dissolves the fibrin aiding the spread allowing for the ability to rest drugs ().
In toxin mechanism, S. aureus has the ability to secrete exotoxins which are linked to various specific
diseases. Superantigens causes nausea, vomiting, diarrhea and abdominal pain. The exfoliative
toxins have the ability to cause staphylococcal scalded skin syndrome. Biofilms are an essential to
group of microorganism which has the ability to grow in wet surfaces. S. aureus biofilm posses the
ability to be resistant to antibiotic management and has the ability to hosts immune response.
Incurring effect entails less effectiveness for S. aureus due to the underlying distribution of antigen
(Archer et al., 2011).
The underlying carriage factors have been a fundamental avenue especially in hospital-
acquired infections and community-acquired infections. Studies have demonstrated that S. aureus is
often present in the skin of the host while a large portion is found in the nasal passage (). The
observed ability for harbouring of the S. aureus has been shown to be demonstrated by weakened
immune systems and ability to evade the innate immunity of the hosts ().
The relevance of S. aureus is an essential aspect. The increased risks in health care settings
have been linked to increased health care cost and longer hospital stay (Nelson et al., 2018). In the
health care settings, it has been a key issue of concern whether in hospitals or medical laboratories.
S. aureus is often transmitted in hospital care settings leading to an immense danger on both the
health care staff and the engaged in testing and diagnosis in laboratory settings. For this purpose the
need to conduct a population-based study among college studies on the positive occurrence of S.
aureus underlying risks factors is essential.
Materials and Methods
This cross-sectional study was conducted among RMIT university students who have been
on the campus for a period of three years in different semesters from 2017 to 2019 enrolled in both
semesters. A total of 909 studies were tested in the study. Nasal sampling was undertaken through
sterile swabs inserted into nostrils for about 1.5 cm in depth and rotated in the nostrils. The swabs
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
4
were then the culture in Mannitol salt agar and incubated to about 24 hours at 37 degrees. The
yellow colonies formed showed the presence of S. aureus due to the fermentation. Further isolation
of the yellow colonies was undertaken, colonies demonstrating positive reaction gram stain,
coagulase and catalyze test were categorized as S. aureus positives (Microbiology Teaching Team,
2020).
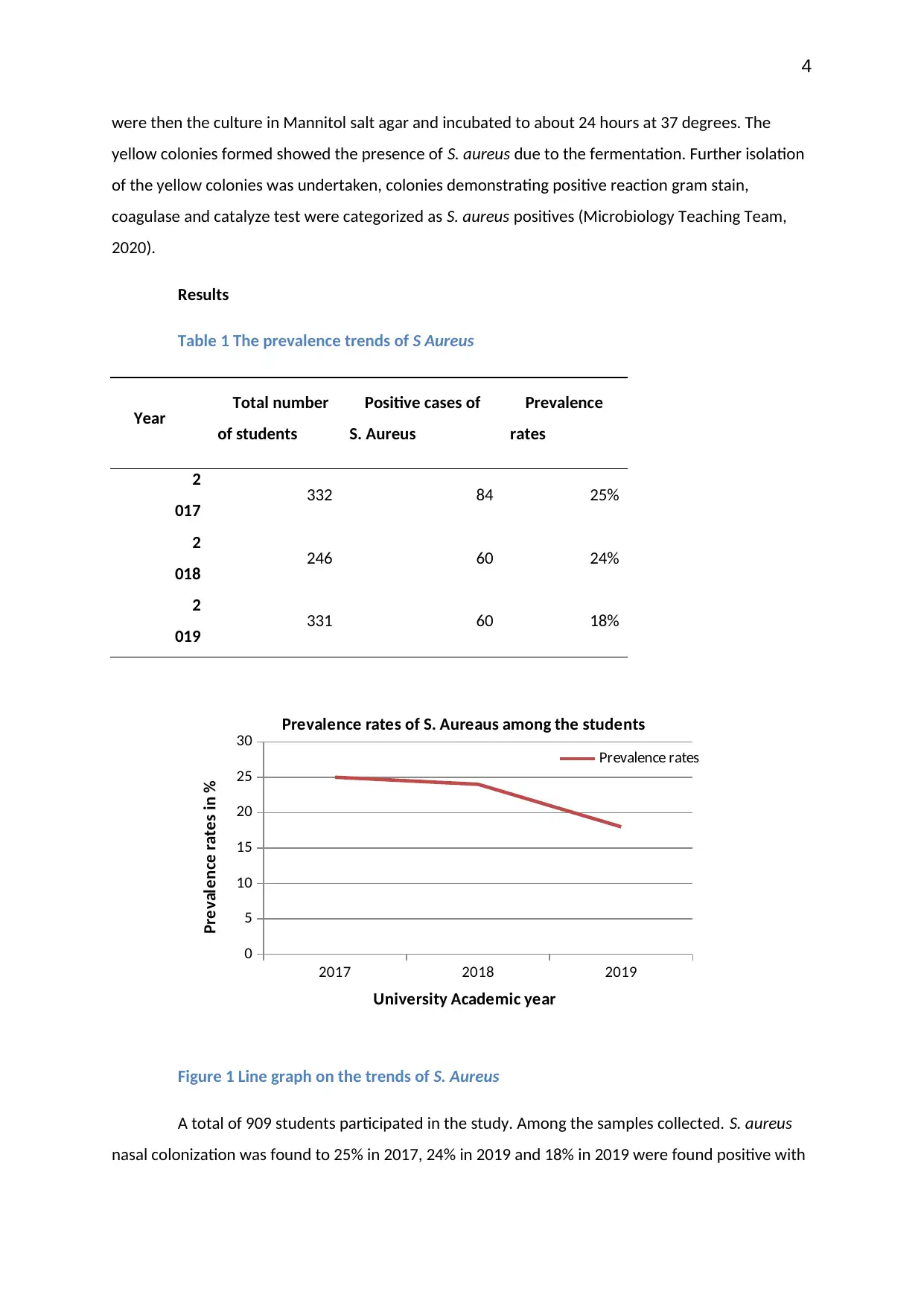
Results
Table 1 The prevalence trends of S Aureus
Year Total number
of students
Positive cases of
S. Aureus
Prevalence
rates
2
017 332 84 25%
2
018 246 60 24%
2
019 331 60 18%
2017 2018 2019
0
5
10
15
20
25
30
Prevalence rates of S. Aureaus among the students
Prevalence rates
University Academic year
Prevalence rates in %
Figure 1 Line graph on the trends of S. Aureus
A total of 909 students participated in the study. Among the samples collected. S. aureus
nasal colonization was found to 25% in 2017, 24% in 2019 and 18% in 2019 were found positive with
were then the culture in Mannitol salt agar and incubated to about 24 hours at 37 degrees. The
yellow colonies formed showed the presence of S. aureus due to the fermentation. Further isolation
of the yellow colonies was undertaken, colonies demonstrating positive reaction gram stain,
coagulase and catalyze test were categorized as S. aureus positives (Microbiology Teaching Team,
2020).
Results
Table 1 The prevalence trends of S Aureus
Year Total number
of students
Positive cases of
S. Aureus
Prevalence
rates
2
017 332 84 25%
2
018 246 60 24%
2
019 331 60 18%
2017 2018 2019
0
5
10
15
20
25
30
Prevalence rates of S. Aureaus among the students
Prevalence rates
University Academic year
Prevalence rates in %
Figure 1 Line graph on the trends of S. Aureus
A total of 909 students participated in the study. Among the samples collected. S. aureus
nasal colonization was found to 25% in 2017, 24% in 2019 and 18% in 2019 were found positive with
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
S. aureus. These carriage rates demonstrated a confirmation based on the Mannitol positive on MSA,
gram-positive cocci and positive tube coagulase.
Discussion
The trends observed in the past three years demonstrate that in 2017 there 25% of
positive carriage rates of S. aureus, while in 2018 and 2019 24% and 18% were recorded
respectively. The trend signifies lower prevalence rates from the previous year’s indicating decline in
the positive carriage among the students at RMIT University.
The global prevalence studies have demonstrated prevalence rates of between 20%-30%,
(Imam et al., 2019). Longitudinal trends across the world further have shown that that population
incidence ranged from between 10 to 30 per 100,000 person-years demonstrating a prevalence of
between 0.001% and 0.003%. In a study on community-based acquired infections in Thailand
demonstrated incidence levels of 2.5 per 100,000 person-years have been showing to have S.aureus
(Kanoksi et al., 2013). In a similar study conducted in India by Pung et al., (2014) among students at
the Faculty of Medicine and Health Sciences, University Putra Malaysia showed that about 65% of
the students were positive carriers. This is quite higher compared to the results obtained in our
study. The declining rates of the prevalence signal a positive trend in infection levels.
Various factors have been demonstrated to affect the rates of disease acquisition among
student base populations and community settings. Demographic factors such as gender and ethnicity
have been shown to have an effect on nasal carriage trends (Chen et al., 2013). Potential risks
factors such as medical history have been reported for S. aureus due to underlying states of health,
lifestyle and social-economic factors which often vary with respect to different climatic conditions
aiding the colonization state and transmission of the S.aureus (Schmid et al., 2013).
The samples in this study were collected once in monthly interval hence allowing for the
short transient pattern. This demonstrated a trend line over the years with declining rates across the
years assessed. This could have an effect on the prolong colonization to a certain extent. Further lack
of a standardized method of undertaking nostril swabs could have a hindrance factor of getting
actual prevalent trends. Further, the difference in the coagulase tests could have impacted on the
accuracy of positive S.aerius among the students.
Conclusion
This study aimed at investigating the prevalence rates of Staphylococcus aureus positive
carriers. The overall impact of the positive cases in the community-acquired infections demonstrates
S. aureus. These carriage rates demonstrated a confirmation based on the Mannitol positive on MSA,
gram-positive cocci and positive tube coagulase.
Discussion
The trends observed in the past three years demonstrate that in 2017 there 25% of
positive carriage rates of S. aureus, while in 2018 and 2019 24% and 18% were recorded
respectively. The trend signifies lower prevalence rates from the previous year’s indicating decline in
the positive carriage among the students at RMIT University.
The global prevalence studies have demonstrated prevalence rates of between 20%-30%,
(Imam et al., 2019). Longitudinal trends across the world further have shown that that population
incidence ranged from between 10 to 30 per 100,000 person-years demonstrating a prevalence of
between 0.001% and 0.003%. In a study on community-based acquired infections in Thailand
demonstrated incidence levels of 2.5 per 100,000 person-years have been showing to have S.aureus
(Kanoksi et al., 2013). In a similar study conducted in India by Pung et al., (2014) among students at
the Faculty of Medicine and Health Sciences, University Putra Malaysia showed that about 65% of
the students were positive carriers. This is quite higher compared to the results obtained in our
study. The declining rates of the prevalence signal a positive trend in infection levels.
Various factors have been demonstrated to affect the rates of disease acquisition among
student base populations and community settings. Demographic factors such as gender and ethnicity
have been shown to have an effect on nasal carriage trends (Chen et al., 2013). Potential risks
factors such as medical history have been reported for S. aureus due to underlying states of health,
lifestyle and social-economic factors which often vary with respect to different climatic conditions
aiding the colonization state and transmission of the S.aureus (Schmid et al., 2013).
The samples in this study were collected once in monthly interval hence allowing for the
short transient pattern. This demonstrated a trend line over the years with declining rates across the
years assessed. This could have an effect on the prolong colonization to a certain extent. Further lack
of a standardized method of undertaking nostril swabs could have a hindrance factor of getting
actual prevalent trends. Further, the difference in the coagulase tests could have impacted on the
accuracy of positive S.aerius among the students.
Conclusion
This study aimed at investigating the prevalence rates of Staphylococcus aureus positive
carriers. The overall impact of the positive cases in the community-acquired infections demonstrates

6
an underlying trend leading to increased rates of Staphylococcus aureus diagnosis among the
population. Despite this, the trends over the years showed a declining demonstrating decrease in
rates of infection among the population signifying improves positive health avenues. Fundamental
techniques to further reduce the prevalence rates entails improved hand hygiene practices, reducing
contacts with wounds and bandages among patients in health care settings and practising a no
sharing approach on personal items such as clothing and cosmetics especially in institution-based
settings.
an underlying trend leading to increased rates of Staphylococcus aureus diagnosis among the
population. Despite this, the trends over the years showed a declining demonstrating decrease in
rates of infection among the population signifying improves positive health avenues. Fundamental
techniques to further reduce the prevalence rates entails improved hand hygiene practices, reducing
contacts with wounds and bandages among patients in health care settings and practising a no
sharing approach on personal items such as clothing and cosmetics especially in institution-based
settings.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
References
Archer, N. K., Mazaitis, M. J., Costerton, J. W., Leid, J. G., Powers, M. E., & Shirtliff, M. E. (2011).
Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence,
2(5), 445-459. doi: 10.4161/viru.2.5.17724.
Boan, P., Tan, H. L., Pearson, J., Coombs, G., Heath, C. H., & Robinson, J. O. (2015). Epidemiological,
clinical, outcome and antibiotic susceptibility differences between PVL positive and PVL
negative Staphylococcus aureus infections in Western Australia: a case control study. BMC
infectious diseases, 15(1), 10. doi: 10.1186/s12879-014-0742-6.
Buchan, K. D., Foster, S. J., & Renshaw, S. A. (2019). Staphylococcus aureus: setting its sights on the
human innate immune system. Microbiology, 165(4), 367-385. doi: 10.1099/mic.0.000759.
Coombs, G. W., Nimmo, G. R., Daley, D. A., Le, T. T., Pearson, J. C., Tan, H. L., ... & Turnidge, J. D.
(2014). Australian Staphylococcus aureus sepsis outcome programme annual report, 2013.
Communicable diseases intelligence quarterly report, 38(4), E309-E319.
https://europepmc.org/article/med/25631593.
Costa, E. M., Silva, S., Tavaria, F. K., & Pintado, M. M. (2017). Insights into chitosan antibiofilm
activity against methicillin‐resistant Staphylococcus aureus. Journal of applied microbiology,
122(6), 1547-1557. doi: 10.1111/jam.13457.
Imam, N., Tempone, S., Armstrong, P. K., McCann, R., Johnson, S., Worth, L. J., & Richards, M. J.
(2019). Increased incidence of community‐associated Staphylococcus aureus bloodstream
infections in Victoria and Western Australia, 2011–2016. The Medical Journal of Australia,
210(2), 87-88. doi: 10.5694/mja2.12057.
Kanoksil, M., Jatapai, A., Peacock, S. J., & Limmathurotsakul, D. (2013). Epidemiology, microbiology
and mortality associated with community-acquired bacteremia in northeast Thailand: a
multicenter surveillance study. PloS one, 8(1). https://doi.org/10.1371/journal.pone.0054714
Microbiology Teaching Team. (2020). Microbiology 1BIOL2158/BIOL2173, Laboratory
Manual,Semester 1. RMIT University.
http://www1.rmit.edu.au/programs/structure/bp231p10ausbu.
Nelson, R. E., Jones, M., Liu, C. F., Samore, M. H., Evans, M. E., Graves, N., ... & Rubin, M. A. (2015).
The impact of healthcare-associated methicillin-resistant Staphylococcus aureus infections on
References
Archer, N. K., Mazaitis, M. J., Costerton, J. W., Leid, J. G., Powers, M. E., & Shirtliff, M. E. (2011).
Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence,
2(5), 445-459. doi: 10.4161/viru.2.5.17724.
Boan, P., Tan, H. L., Pearson, J., Coombs, G., Heath, C. H., & Robinson, J. O. (2015). Epidemiological,
clinical, outcome and antibiotic susceptibility differences between PVL positive and PVL
negative Staphylococcus aureus infections in Western Australia: a case control study. BMC
infectious diseases, 15(1), 10. doi: 10.1186/s12879-014-0742-6.
Buchan, K. D., Foster, S. J., & Renshaw, S. A. (2019). Staphylococcus aureus: setting its sights on the
human innate immune system. Microbiology, 165(4), 367-385. doi: 10.1099/mic.0.000759.
Coombs, G. W., Nimmo, G. R., Daley, D. A., Le, T. T., Pearson, J. C., Tan, H. L., ... & Turnidge, J. D.
(2014). Australian Staphylococcus aureus sepsis outcome programme annual report, 2013.
Communicable diseases intelligence quarterly report, 38(4), E309-E319.
https://europepmc.org/article/med/25631593.
Costa, E. M., Silva, S., Tavaria, F. K., & Pintado, M. M. (2017). Insights into chitosan antibiofilm
activity against methicillin‐resistant Staphylococcus aureus. Journal of applied microbiology,
122(6), 1547-1557. doi: 10.1111/jam.13457.
Imam, N., Tempone, S., Armstrong, P. K., McCann, R., Johnson, S., Worth, L. J., & Richards, M. J.
(2019). Increased incidence of community‐associated Staphylococcus aureus bloodstream
infections in Victoria and Western Australia, 2011–2016. The Medical Journal of Australia,
210(2), 87-88. doi: 10.5694/mja2.12057.
Kanoksil, M., Jatapai, A., Peacock, S. J., & Limmathurotsakul, D. (2013). Epidemiology, microbiology
and mortality associated with community-acquired bacteremia in northeast Thailand: a
multicenter surveillance study. PloS one, 8(1). https://doi.org/10.1371/journal.pone.0054714
Microbiology Teaching Team. (2020). Microbiology 1BIOL2158/BIOL2173, Laboratory
Manual,Semester 1. RMIT University.
http://www1.rmit.edu.au/programs/structure/bp231p10ausbu.
Nelson, R. E., Jones, M., Liu, C. F., Samore, M. H., Evans, M. E., Graves, N., ... & Rubin, M. A. (2015).
The impact of healthcare-associated methicillin-resistant Staphylococcus aureus infections on
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
post-discharge healthcare costs and utilization. infection control & hospital epidemiology,
36(5), 534-542. DOI: 10.1111/1475-6773.13063
Pung, H. P., Azis, M. N., Rachman, A. A., Zakaria, Z. A., & Desa, M. M. (2017). Presence of methicillin
resistance and heteroresistance among Coagulase Negative Staphylococci (CoNS) isolates
obtained from Health Sciences students at a Public University. Tropical Biomedicine, 34(1), 84-
88. https://www.cabdirect.org/cabdirect/abstract/20173266512.
Schmid, H., Romanos, A., Schiffl, H., & Lederer, S. R. (2013). Persistent nasal methicillin-resistant
Staphylococcus aureus carriage in hemodialysis outpatients: a predictor of worse outcome.
BMC nephrology, 14(1), 93. https://bmcnephrol.biomedcentral.com/articles/10.1186/1471-
2369-14-93.
Tong, S. Y. C., Varrone, L., Chatfield, M. D., Beaman, M., & Giffard, P. M. (2015). Progressive increase
in community-associated methicillin-resistant Staphylococcus aureus in Indigenous
populations in northern Australia from 1993 to 2012. Epidemiology & Infection, 143(7), 1519-
1523. DOI: https://doi.org/10.1017/S0950268814002611
Williamson, D. A., Coombs, G. W., & Nimmo, G. R. (2014). Staphylococcus aureus ‘Down Under’:
Contemporary epidemiology of S. aureus in Australia, New Zealand, and the south west
Pacific. Clinical Microbiology and Infection, 20(7), 597-604. doi: 10.1111/1469-0691.12702.
Epub 2014 Jul 12.
post-discharge healthcare costs and utilization. infection control & hospital epidemiology,
36(5), 534-542. DOI: 10.1111/1475-6773.13063
Pung, H. P., Azis, M. N., Rachman, A. A., Zakaria, Z. A., & Desa, M. M. (2017). Presence of methicillin
resistance and heteroresistance among Coagulase Negative Staphylococci (CoNS) isolates
obtained from Health Sciences students at a Public University. Tropical Biomedicine, 34(1), 84-
88. https://www.cabdirect.org/cabdirect/abstract/20173266512.
Schmid, H., Romanos, A., Schiffl, H., & Lederer, S. R. (2013). Persistent nasal methicillin-resistant
Staphylococcus aureus carriage in hemodialysis outpatients: a predictor of worse outcome.
BMC nephrology, 14(1), 93. https://bmcnephrol.biomedcentral.com/articles/10.1186/1471-
2369-14-93.
Tong, S. Y. C., Varrone, L., Chatfield, M. D., Beaman, M., & Giffard, P. M. (2015). Progressive increase
in community-associated methicillin-resistant Staphylococcus aureus in Indigenous
populations in northern Australia from 1993 to 2012. Epidemiology & Infection, 143(7), 1519-
1523. DOI: https://doi.org/10.1017/S0950268814002611
Williamson, D. A., Coombs, G. W., & Nimmo, G. R. (2014). Staphylococcus aureus ‘Down Under’:
Contemporary epidemiology of S. aureus in Australia, New Zealand, and the south west
Pacific. Clinical Microbiology and Infection, 20(7), 597-604. doi: 10.1111/1469-0691.12702.
Epub 2014 Jul 12.
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





