1807NRS: Safe Administration of Medications Assignment
VerifiedAdded on 2022/09/16
|19
|3990
|17
Homework Assignment
AI Summary
This assignment, completed by a student, addresses the safe administration of medications (SAM) within a healthcare context. It comprehensively covers the regulation of medications in Australia, the principles of pharmacokinetics and pharmacodynamics, and the roles and responsibilities of various healthcare professionals (doctors, RNs, and pharmacists) in the medication cycle. The assignment delves into the various factors that contribute to medication errors, including high workloads, inexperienced staff, and environmental factors, offering insights into error-producing conditions and preventative strategies. It also explores the medication management cycle, and contrasts person-centered and system approaches to medication error analysis. Specific topics include the first-pass effect, the use of Glyceryl Trinitrate, and the Swiss cheese model for understanding system failures. The assignment aims to demonstrate an understanding of medication safety and error reduction within the nursing practice.

Running Head: SAM
0
Safe Administration of Medicines
Question answers
student
8/27/2019
0
Safe Administration of Medicines
Question answers
student
8/27/2019
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SAM
1
Table of Contents
Ans. 1...........................................................................................................................................................2
Ans. 2.......................................................................................................................................................2
Ans. 3.......................................................................................................................................................3
Ans. 4.......................................................................................................................................................3
Ans. 5.......................................................................................................................................................4
Ans. 6.......................................................................................................................................................4
Ans. 7.......................................................................................................................................................5
Ans. 8.......................................................................................................................................................7
Ans. 9.....................................................................................................................................................10
References.................................................................................................................................................12
1
Table of Contents
Ans. 1...........................................................................................................................................................2
Ans. 2.......................................................................................................................................................2
Ans. 3.......................................................................................................................................................3
Ans. 4.......................................................................................................................................................3
Ans. 5.......................................................................................................................................................4
Ans. 6.......................................................................................................................................................4
Ans. 7.......................................................................................................................................................5
Ans. 8.......................................................................................................................................................7
Ans. 9.....................................................................................................................................................10
References.................................................................................................................................................12

SAM
2
Ans. 1
(A, B)
1. Pre-market assessment; this helps in evaluating and assessing the risks associated with
the therapeutic products (Ghosh, Skinner & Ferguson, 2006).
2. Post-market monitoring and enforcement of standards; this helps in identifying and
assessing any risk posed by the product after released in the market (Kumari, et al., 2016).
3. Licensing of Australian manufacturers and verifying overseas manufacturers'
compliance with the same standards (Kulkarni, 2017) as their Australian counterparts; this
helps in the manufacturing of risk free and good quality products by approving the selected
companies only (Department of Health: Therapeutic Goods administration, 2019).
Ans. 2
Phase 1: This particular phase is conducted to define effects of the medicine or medical device
on human involving how it is absorbed, metabolized, and eliminated from the body (Friedman et
al., 2015).
Phase 2: This 2nd phase of drug testing includes up to several 100 patients. This permits
researchers to deliver the pharmaceutical organization and the FDA with relative information
about the comparative safety and efficiency of the novel drug (Pallmann et al., 2018).
Phase 3: This is the large-scale examination, which delivers the knowledge of the efficiency of
the medicine or device, the advantages and the variety of probable side effects (Piantadosi,
2017).
2
Ans. 1
(A, B)
1. Pre-market assessment; this helps in evaluating and assessing the risks associated with
the therapeutic products (Ghosh, Skinner & Ferguson, 2006).
2. Post-market monitoring and enforcement of standards; this helps in identifying and
assessing any risk posed by the product after released in the market (Kumari, et al., 2016).
3. Licensing of Australian manufacturers and verifying overseas manufacturers'
compliance with the same standards (Kulkarni, 2017) as their Australian counterparts; this
helps in the manufacturing of risk free and good quality products by approving the selected
companies only (Department of Health: Therapeutic Goods administration, 2019).
Ans. 2
Phase 1: This particular phase is conducted to define effects of the medicine or medical device
on human involving how it is absorbed, metabolized, and eliminated from the body (Friedman et
al., 2015).
Phase 2: This 2nd phase of drug testing includes up to several 100 patients. This permits
researchers to deliver the pharmaceutical organization and the FDA with relative information
about the comparative safety and efficiency of the novel drug (Pallmann et al., 2018).
Phase 3: This is the large-scale examination, which delivers the knowledge of the efficiency of
the medicine or device, the advantages and the variety of probable side effects (Piantadosi,
2017).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

SAM
3
Phase 4: The Pharmaceutical organizations can compare a new drug with other different drugs
already available in the market; to screen a drug's lasting efficiency and effects on a diseased
person’s quality of life; and to identify the cost-effectiveness of the drug therapy comparative to
other customary and new treatments (Wright, 2017).
Ans. 3
Pharmacokinetics is presently described as the study of the time taken by the drug for absorption,
circulation, metabolism, and elimination (Wagner, 2018). The Clinical pharmacokinetics is the
use of principles of pharmacokinetic to the harmless and effective therapeutic controlling of
drugs in a particular patient. Primary goals of medical pharmacokinetics comprise increasing
efficacy and reducing toxicity of drug therapy (Sime et al., 2018).
Pharmacodynamics defined as the association between concentration of the drug at the place of
action and the subsequent effect, counting the time course and concentration of therapeutic and
negative reaction (Kamath, 2016). The impacts of a drug existing at the location of action are
identified by the binding of drug with the receptor. For instance, Receptors might be available on
neurons in the CNS to reduce pain sensation (Weerink et al., 2017).
Ans. 4
First-pass effect is when a drug administered to the patient, enters his or her liver and goes
through widespread biotransformation and therefore reducing the concentration quickly before it
reaches to its target (Kamath, 2016). It takes place most usually when a drug is provided orally.
Later the drug is absorbed in patient’s GIT and reaches the portal circulation beforehand entering
patient’s systemic circulation (Wang, et al., 2017). Through the portal flow it transports to the
liver where certain drugs undertake widespread biotransformation and the concentration of the
3
Phase 4: The Pharmaceutical organizations can compare a new drug with other different drugs
already available in the market; to screen a drug's lasting efficiency and effects on a diseased
person’s quality of life; and to identify the cost-effectiveness of the drug therapy comparative to
other customary and new treatments (Wright, 2017).
Ans. 3
Pharmacokinetics is presently described as the study of the time taken by the drug for absorption,
circulation, metabolism, and elimination (Wagner, 2018). The Clinical pharmacokinetics is the
use of principles of pharmacokinetic to the harmless and effective therapeutic controlling of
drugs in a particular patient. Primary goals of medical pharmacokinetics comprise increasing
efficacy and reducing toxicity of drug therapy (Sime et al., 2018).
Pharmacodynamics defined as the association between concentration of the drug at the place of
action and the subsequent effect, counting the time course and concentration of therapeutic and
negative reaction (Kamath, 2016). The impacts of a drug existing at the location of action are
identified by the binding of drug with the receptor. For instance, Receptors might be available on
neurons in the CNS to reduce pain sensation (Weerink et al., 2017).
Ans. 4
First-pass effect is when a drug administered to the patient, enters his or her liver and goes
through widespread biotransformation and therefore reducing the concentration quickly before it
reaches to its target (Kamath, 2016). It takes place most usually when a drug is provided orally.
Later the drug is absorbed in patient’s GIT and reaches the portal circulation beforehand entering
patient’s systemic circulation (Wang, et al., 2017). Through the portal flow it transports to the
liver where certain drugs undertake widespread biotransformation and the concentration of the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SAM
4
drug is reduced (Svennebring, 2015). Therefore it is the portion of lost medicine throughout the
course of absorption commonly associated with the liver (Tian et al., 2016).
Ans. 5
A) Glyceryl Trinitrate 600 microgram is designed to liquefy rapidly underneath the tongue.
This particular area of the human mouth has a great supply of the blood vessels that
permits the drug to be quickly absorbed (Abbas, Elsherbini & Shaldam, 2019). Glyceryl
Trinitrate 600 microgram administered in this manner is used to provide quick release
from the pain of associated with angina attack. Patients are recommended to not to
swallow, suck or chew the medicine like other drugs (Thompson, A. (2016).
B)
The patient must be educated about placing the medicine beneath the tongue only
Ms. Scott should be informed to keep the drug with her always in case she needs to
take one.
They must contact to the health professional like nurses if experience any side effect
after taking this drug and must avoid consuming alcohol as it can enhance the side
effects of this drug like feeling faintness, or dizziness (Scutt et al., 2018).
the dosage must be repeated after 5 minutes if the symptoms are not resolved
It is best to advice Ms. Scott to sit down before when taking the medicine as
otherwise it might make her feel dizzy (Omar, Jasudass & Saad, 2015).
4
drug is reduced (Svennebring, 2015). Therefore it is the portion of lost medicine throughout the
course of absorption commonly associated with the liver (Tian et al., 2016).
Ans. 5
A) Glyceryl Trinitrate 600 microgram is designed to liquefy rapidly underneath the tongue.
This particular area of the human mouth has a great supply of the blood vessels that
permits the drug to be quickly absorbed (Abbas, Elsherbini & Shaldam, 2019). Glyceryl
Trinitrate 600 microgram administered in this manner is used to provide quick release
from the pain of associated with angina attack. Patients are recommended to not to
swallow, suck or chew the medicine like other drugs (Thompson, A. (2016).
B)
The patient must be educated about placing the medicine beneath the tongue only
Ms. Scott should be informed to keep the drug with her always in case she needs to
take one.
They must contact to the health professional like nurses if experience any side effect
after taking this drug and must avoid consuming alcohol as it can enhance the side
effects of this drug like feeling faintness, or dizziness (Scutt et al., 2018).
the dosage must be repeated after 5 minutes if the symptoms are not resolved
It is best to advice Ms. Scott to sit down before when taking the medicine as
otherwise it might make her feel dizzy (Omar, Jasudass & Saad, 2015).

SAM
5
Ans. 6
The main role of doctors is to prescribe the drug according to the healthcare needs of the
patient (Liu, Gerdtz, & Manias, 2016).
They undertake the patient consultation and perform physical examination before
prescribing the drug
They make the complex decision related to the administration of the drug (McGavock,
2017).
Role of RN
They must assess the patient for any allergies associated with any content of the drug
They educate the patient and their family about the do’s and don’ts of the drug
They must also ensure the five rights of medication administration (Venables & Gunnell,
2018).
Pharmacist
They read the prescription of the doctor and provide the right drug to the patient so that
medication error can be avoided
They educate the patient about safe storage of the drug
They can also inform the patient and their family about drug interaction associated with
the particular drug (McGavock, 2017).
5
Ans. 6
The main role of doctors is to prescribe the drug according to the healthcare needs of the
patient (Liu, Gerdtz, & Manias, 2016).
They undertake the patient consultation and perform physical examination before
prescribing the drug
They make the complex decision related to the administration of the drug (McGavock,
2017).
Role of RN
They must assess the patient for any allergies associated with any content of the drug
They educate the patient and their family about the do’s and don’ts of the drug
They must also ensure the five rights of medication administration (Venables & Gunnell,
2018).
Pharmacist
They read the prescription of the doctor and provide the right drug to the patient so that
medication error can be avoided
They educate the patient about safe storage of the drug
They can also inform the patient and their family about drug interaction associated with
the particular drug (McGavock, 2017).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

SAM
6
Ans. 7
Medication management cycle
1. Decision to treat and prescribe: the prescriber should assess to the accurate, complete
and updated consumer related information and include the patient in the decision making
process
2. Record of the medicine order/prescription: the information of the prescription must be
recorded to transfer to other person in the chain. There should be a proper communication
between patient, nurses, and pharmacist, and physician (Lindblad, Flink & Ekstedt,
2017).
3. Review of the medication order/prescription: review provides important safety for the
patient, it ensures the compliance with the requirement of legislation, optimal medicinal
use, verify intentions, and administration.
4. Issue of medicine: issuing a drug involves the processes of dispensing, developing, or
transferring, and is commonly undertaken by the pharmacist or the endorsed nurses. The
exact medicine should be developed or selected, labelled completely and clearly, and a
record of an issue made.
5. Provision of the medicine information: Provision of patient -specific drug information,
counting how to stock and correctly use medications, and improves safety (Coffman,
Vanderveen, Lee & Schlotterbeck, 2017).
6. Distribution and storage: once issued, the medicines are supplied to the care delivery
areas like ward within the health care facility.
7. Administration of the medicine: this particular step includes the reassessment of need,
choice of exact medicine, preparation and administration for the right person every time.
6
Ans. 7
Medication management cycle
1. Decision to treat and prescribe: the prescriber should assess to the accurate, complete
and updated consumer related information and include the patient in the decision making
process
2. Record of the medicine order/prescription: the information of the prescription must be
recorded to transfer to other person in the chain. There should be a proper communication
between patient, nurses, and pharmacist, and physician (Lindblad, Flink & Ekstedt,
2017).
3. Review of the medication order/prescription: review provides important safety for the
patient, it ensures the compliance with the requirement of legislation, optimal medicinal
use, verify intentions, and administration.
4. Issue of medicine: issuing a drug involves the processes of dispensing, developing, or
transferring, and is commonly undertaken by the pharmacist or the endorsed nurses. The
exact medicine should be developed or selected, labelled completely and clearly, and a
record of an issue made.
5. Provision of the medicine information: Provision of patient -specific drug information,
counting how to stock and correctly use medications, and improves safety (Coffman,
Vanderveen, Lee & Schlotterbeck, 2017).
6. Distribution and storage: once issued, the medicines are supplied to the care delivery
areas like ward within the health care facility.
7. Administration of the medicine: this particular step includes the reassessment of need,
choice of exact medicine, preparation and administration for the right person every time.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SAM
7
8. Monitor for the response: the health professional receive the medicine associated
response of the consumer to identify the efficacy and side effects of the drug to the
particular patient (Neyarapally & Smith, 2017).
9. Transfer of verified information: information on the drug and related treatment plan is
essential to examine effectiveness, assist with the upcoming decision about the treatment,
and enable the safe transfer of patient care, particularly when other healthcare
professional is involved in the current patient care. This involves the present medicine
regimen, quantity of the drug, and change of the treatment during the patient care
episodes (Blanchard et al., 2016).
Causes of medication error
1. High staff workload and fatigue can leads to wrong prescription and treatment
2. Lack of effective communication between the team members and patient
3. In-experienced and inadequately trained healthcare staff can also impacts the safe
administration of the drug and leads to medicinal error
4. Confusion in the nomenclature, packaging, and labelling of the drug
5. Lack of effective policies and procedures associated with the drugs
6. Increased number or the quantity of medicines per patient (Neyarapally & Smith, 2017).
7. Environmental factor like poor lighting, extra noise, frequent interruptions, and lack of
facilities for proper communication with the patient.
8. Frequency and the complexity of the calculation required to prescribe, distribute or
administer the medicines (Coffman, Vanderveen, Lee & Schlotterbeck, 2017).
7
8. Monitor for the response: the health professional receive the medicine associated
response of the consumer to identify the efficacy and side effects of the drug to the
particular patient (Neyarapally & Smith, 2017).
9. Transfer of verified information: information on the drug and related treatment plan is
essential to examine effectiveness, assist with the upcoming decision about the treatment,
and enable the safe transfer of patient care, particularly when other healthcare
professional is involved in the current patient care. This involves the present medicine
regimen, quantity of the drug, and change of the treatment during the patient care
episodes (Blanchard et al., 2016).
Causes of medication error
1. High staff workload and fatigue can leads to wrong prescription and treatment
2. Lack of effective communication between the team members and patient
3. In-experienced and inadequately trained healthcare staff can also impacts the safe
administration of the drug and leads to medicinal error
4. Confusion in the nomenclature, packaging, and labelling of the drug
5. Lack of effective policies and procedures associated with the drugs
6. Increased number or the quantity of medicines per patient (Neyarapally & Smith, 2017).
7. Environmental factor like poor lighting, extra noise, frequent interruptions, and lack of
facilities for proper communication with the patient.
8. Frequency and the complexity of the calculation required to prescribe, distribute or
administer the medicines (Coffman, Vanderveen, Lee & Schlotterbeck, 2017).

SAM
8
Ans. 8
A)
8
Ans. 8
A)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
SAM
9
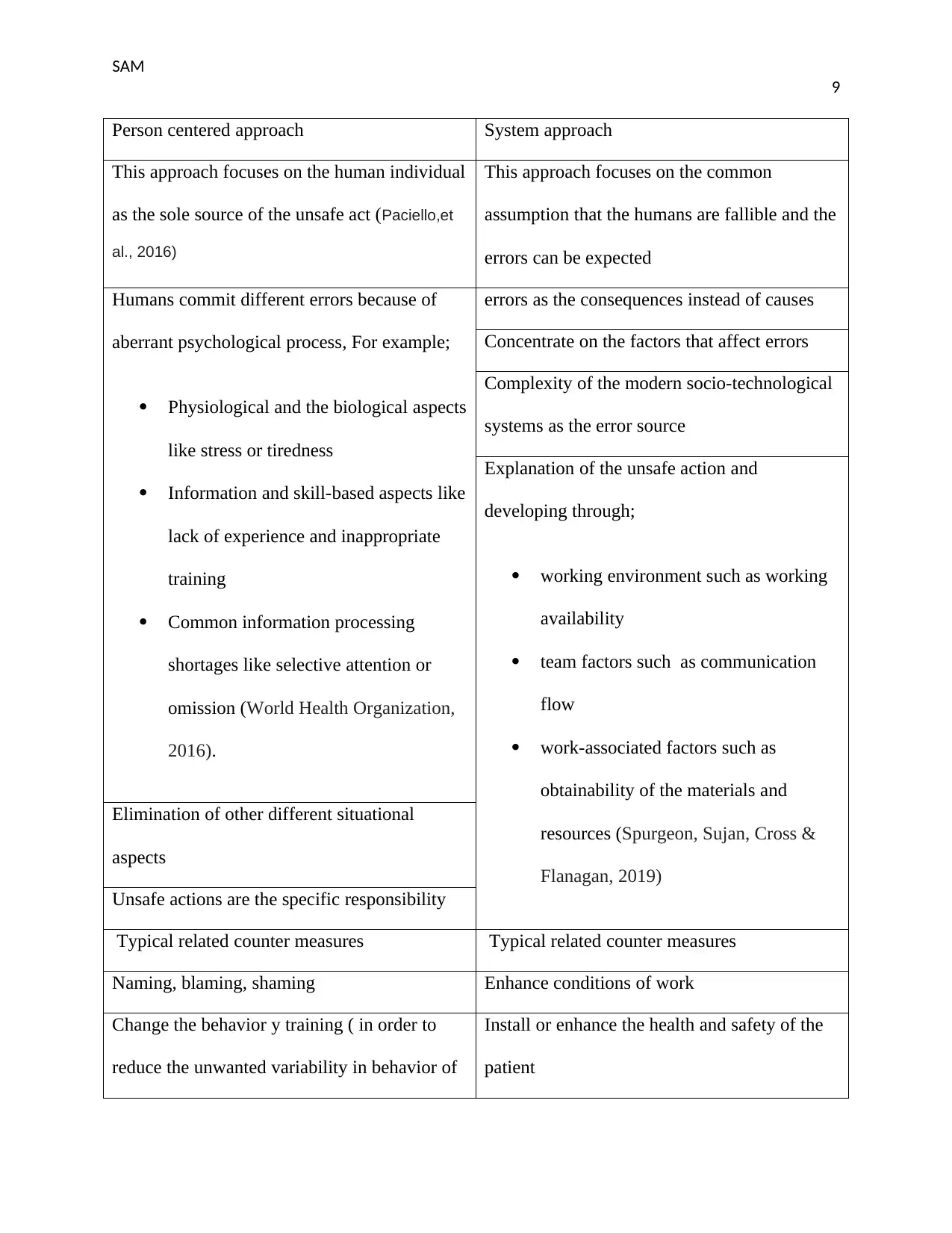
Person centered approach System approach
This approach focuses on the human individual
as the sole source of the unsafe act (Paciello,et
al., 2016)
This approach focuses on the common
assumption that the humans are fallible and the
errors can be expected
Humans commit different errors because of
aberrant psychological process, For example;
Physiological and the biological aspects
like stress or tiredness
Information and skill-based aspects like
lack of experience and inappropriate
training
Common information processing
shortages like selective attention or
omission (World Health Organization,
2016).
errors as the consequences instead of causes
Concentrate on the factors that affect errors
Complexity of the modern socio-technological
systems as the error source
Explanation of the unsafe action and
developing through;
working environment such as working
availability
team factors such as communication
flow
work-associated factors such as
obtainability of the materials and
resources (Spurgeon, Sujan, Cross &
Flanagan, 2019)
Elimination of other different situational
aspects
Unsafe actions are the specific responsibility
Typical related counter measures Typical related counter measures
Naming, blaming, shaming Enhance conditions of work
Change the behavior y training ( in order to
reduce the unwanted variability in behavior of
Install or enhance the health and safety of the
patient
9
Person centered approach System approach
This approach focuses on the human individual
as the sole source of the unsafe act (Paciello,et
al., 2016)
This approach focuses on the common
assumption that the humans are fallible and the
errors can be expected
Humans commit different errors because of
aberrant psychological process, For example;
Physiological and the biological aspects
like stress or tiredness
Information and skill-based aspects like
lack of experience and inappropriate
training
Common information processing
shortages like selective attention or
omission (World Health Organization,
2016).
errors as the consequences instead of causes
Concentrate on the factors that affect errors
Complexity of the modern socio-technological
systems as the error source
Explanation of the unsafe action and
developing through;
working environment such as working
availability
team factors such as communication
flow
work-associated factors such as
obtainability of the materials and
resources (Spurgeon, Sujan, Cross &
Flanagan, 2019)
Elimination of other different situational
aspects
Unsafe actions are the specific responsibility
Typical related counter measures Typical related counter measures
Naming, blaming, shaming Enhance conditions of work
Change the behavior y training ( in order to
reduce the unwanted variability in behavior of
Install or enhance the health and safety of the
patient
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SAM
10
health care professionals and patients
Removes and exchange individuals Change the Material mindset and culture of the
individual or patient
The person approach leftovers the leading
custom in medicine, as to a different place.
From selected viewpoints it has much to praise
it. Blaming persons is emotionally additional
satisfying than pointing institutions.
Individuals are observed as free mediators
capable of selecting between harmless and
unsafe styles of behaviour (Gilbert & Kim,
2018).
Latent disappointments, or errors at the “blunt
end” associated with the system be motivated
to be less obvious, and they are accredited to
wider organizational effects counting but not
restricted to economic restraints, quality
management scheme, structural culture,
distribution of resources, communication, work
procedures, administrative rules, guidelines,
etc. (Newton, Asimakopoulou, Al-Haboubi &
Scambler, 2017).
B) Defences, safeguards, and barriers are the finest ways to stop errors in a healthcare
facility. Every barrier is seen as the slice portion of cheese. In the perfect word, there are
no holes in the barriers. Though, in the actual world, the numerous defences arrange like
slices of the Swiss cheese model. When all the holes arrange in a line, system failure or
error might occur. The barriers can put in place, at the organization level, to prevent the
adverse events. For example two medicines with similar pronunciation can cause
confusion until safeguards are in place to prevent it. Without the safeguards or timely
interventions, for example cascades can compromise the patient safety (Stein & Heiss,
2015).
10
health care professionals and patients
Removes and exchange individuals Change the Material mindset and culture of the
individual or patient
The person approach leftovers the leading
custom in medicine, as to a different place.
From selected viewpoints it has much to praise
it. Blaming persons is emotionally additional
satisfying than pointing institutions.
Individuals are observed as free mediators
capable of selecting between harmless and
unsafe styles of behaviour (Gilbert & Kim,
2018).
Latent disappointments, or errors at the “blunt
end” associated with the system be motivated
to be less obvious, and they are accredited to
wider organizational effects counting but not
restricted to economic restraints, quality
management scheme, structural culture,
distribution of resources, communication, work
procedures, administrative rules, guidelines,
etc. (Newton, Asimakopoulou, Al-Haboubi &
Scambler, 2017).
B) Defences, safeguards, and barriers are the finest ways to stop errors in a healthcare
facility. Every barrier is seen as the slice portion of cheese. In the perfect word, there are
no holes in the barriers. Though, in the actual world, the numerous defences arrange like
slices of the Swiss cheese model. When all the holes arrange in a line, system failure or
error might occur. The barriers can put in place, at the organization level, to prevent the
adverse events. For example two medicines with similar pronunciation can cause
confusion until safeguards are in place to prevent it. Without the safeguards or timely
interventions, for example cascades can compromise the patient safety (Stein & Heiss,
2015).

SAM
11
C) Both the latent conditions and active failures interrelate, making a window for the error
to occur. Latent conditions set the stage for the medicinal error, whereas active failures
incline to be the promoter for the medicinal.
1. Active failures
use of incorrect procedures
not following procedures
not finishing a final system check correctly
2. Latent failures are caused by circumstances such as
scheduling problems
inadequate training,
Lack of resources (Seshia et al., 2018).
Ans. 9
Factors to contribute medicinal error
1. High workload
2. In-experienced staff
3. Confusion with similar name medicines
4. Shift work
5. Environmental factors
High workload: nurses and other health care professional have to perform many tasks
daily and lack of staff in the healthcare facility can put the high workload pressure on available
staff as they have to perform additional tasks, this can ultimately leads to medicinal error. This
11
C) Both the latent conditions and active failures interrelate, making a window for the error
to occur. Latent conditions set the stage for the medicinal error, whereas active failures
incline to be the promoter for the medicinal.
1. Active failures
use of incorrect procedures
not following procedures
not finishing a final system check correctly
2. Latent failures are caused by circumstances such as
scheduling problems
inadequate training,
Lack of resources (Seshia et al., 2018).
Ans. 9
Factors to contribute medicinal error
1. High workload
2. In-experienced staff
3. Confusion with similar name medicines
4. Shift work
5. Environmental factors
High workload: nurses and other health care professional have to perform many tasks
daily and lack of staff in the healthcare facility can put the high workload pressure on available
staff as they have to perform additional tasks, this can ultimately leads to medicinal error. This
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 19
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





